The Key Points
Allokos stated that lirentelimab failed two clinical trials to meet the co-primary endpoints.
The company hopes to identify a way forward for the drug to treat eosinophilic gastro diseases.
Allokos continues to study the drug for other indications.
What happened?
As of Wednesday morning's 10:17 ET, shares of Allakos (NASDAQ :ALLK) were 88% lower. This huge drop occurred after Allakos announced the results of its phase 3 Enigma 2 clinical study evaluating lirentelimab and its phase 2/3 Kryptos phase 2/3 clinical study evaluating lirentelimab.
Allakos stated that both clinical trials met their histologic coprimary endpoints. However, statistically significant improvements in patient-reported symptoms were not achieved by either study.
Scientists are often concerned by their expressions.
What are you waiting for?
Because the co-primary endpoint missedes were not even close, the biotech stock plummeted. This is especially concerning because Allokos has only one pipeline candidate for clinical development, lirentelimab.
Patients who were treated with a placebo in the Enigma 2 trial reported greater absolute changes in their six-symptom total symptoms score (TSS). This was in comparison to patients who received lirentelimab. The Kryptos trial, which involved patients who received a low dose of drug, showed a similar result. The high dose of lirentelimab showed greater improvements in symptoms than patients who received a placebo. However, it was not statistically significant.
The executives at Allakos didn't spin the results as being positive. Robert Alexander, CEO of Allakos, stated that "we are deeply disappointed that their studies failed to achieve their symptomatic endspoints." Craig Paterson, chief medical officer, called the results "surprising" and "disappointing."
What now?
Paterson said that Allokos plans to "at present" continue to analyze the data from the two studies to determine a path forward to lirentelimab's use in the treatment of eosinophilic gastro diseases. The company plans to develop a subcutaneous drug version that targets atopic dermatitis and chronic spontaneous urticaria.
This article is the author's opinion. He or she may not agree with the official recommendation of a Motley Fool premium advisory services. We're motley! We are all different!

 Who was Dror Or, the Israeli father who died as a hostage in the hands of Hamas?
Who was Dror Or, the Israeli father who died as a hostage in the hands of Hamas? “Pay in cash”: at his trial, Donald Trump faced with an embarrassing recording
“Pay in cash”: at his trial, Donald Trump faced with an embarrassing recording Italy: a grandmother accidentally serves a bottle filled with wine to a baby, he has an alcoholic coma
Italy: a grandmother accidentally serves a bottle filled with wine to a baby, he has an alcoholic coma The mysterious skeletons of Hermann Göring's villa
The mysterious skeletons of Hermann Göring's villa Children born thanks to PMA do not have more cancers than others
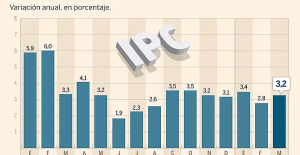
Children born thanks to PMA do not have more cancers than others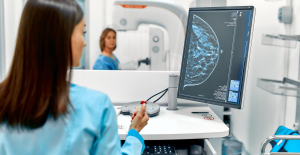 Breast cancer: less than one in two French women follow screening recommendations
Breast cancer: less than one in two French women follow screening recommendations “Dazzling” symptoms, 5,000 deaths per year, non-existent vaccine... What is Lassa fever, a case of which has been identified in Île-de-France?
“Dazzling” symptoms, 5,000 deaths per year, non-existent vaccine... What is Lassa fever, a case of which has been identified in Île-de-France? Sánchez cancels his agenda and considers resigning: "I need to stop and reflect"
Sánchez cancels his agenda and considers resigning: "I need to stop and reflect" Health carpooling, this source of savings which arouses the ire of patients and taxis
Health carpooling, this source of savings which arouses the ire of patients and taxis Tesla Model 3, MG4 and Dacia Spring.... With the end of the ecological bonus, these electric cars produced in China are seeing their sales fall
Tesla Model 3, MG4 and Dacia Spring.... With the end of the ecological bonus, these electric cars produced in China are seeing their sales fall For the 2024 Olympics, Airbnb commits to fighting prostitution in its accommodation
For the 2024 Olympics, Airbnb commits to fighting prostitution in its accommodation “Shrinkflation”: supermarkets obliged to alert their customers from July 1
“Shrinkflation”: supermarkets obliged to alert their customers from July 1 The electro of Justice and the echoes of Portishead
The electro of Justice and the echoes of Portishead 1924 Olympic Games: according to his daughter, the hero of Chariots of Fire was “not a bigot”
1924 Olympic Games: according to his daughter, the hero of Chariots of Fire was “not a bigot” The “German Brothel” in Yvelines: an uncertain future for the ruined residence
The “German Brothel” in Yvelines: an uncertain future for the ruined residence The eye of the INA: when Paul Auster visited Bernard Pivot
The eye of the INA: when Paul Auster visited Bernard Pivot Omoda 7, another Chinese car that could be manufactured in Spain
Omoda 7, another Chinese car that could be manufactured in Spain BYD chooses CA Auto Bank as financial partner in Spain
BYD chooses CA Auto Bank as financial partner in Spain Tesla and Baidu sign key agreement to boost development of autonomous driving
Tesla and Baidu sign key agreement to boost development of autonomous driving Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV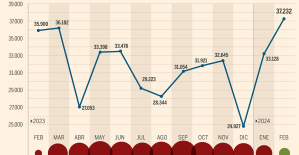 The home mortgage firm rises 3.8% in February and the average interest moderates to 3.33%
The home mortgage firm rises 3.8% in February and the average interest moderates to 3.33%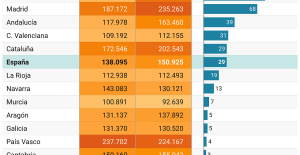 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade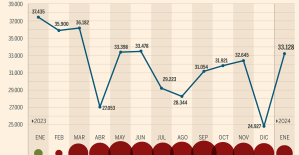 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella Facing Jordan Bardella, the popularity match turns to Gabriel Attal’s advantage
Facing Jordan Bardella, the popularity match turns to Gabriel Attal’s advantage Europeans: a senior official on the National Rally list
Europeans: a senior official on the National Rally list Blockade of Sciences Po: the right denounces a “drift”, the government charges the rebels
Blockade of Sciences Po: the right denounces a “drift”, the government charges the rebels Even on a mission for NATO, the Charles-de-Gaulle remains under French control, Lecornu responds to Mélenchon
Even on a mission for NATO, the Charles-de-Gaulle remains under French control, Lecornu responds to Mélenchon These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Monaco - Clermont: Minamino cornerstone, Fofana essential, the Clermont defense overwhelmed... The tops and the flops
Monaco - Clermont: Minamino cornerstone, Fofana essential, the Clermont defense overwhelmed... The tops and the flops Gymnastics: two gold medals for the Italian Manila Esposito during the European Championships
Gymnastics: two gold medals for the Italian Manila Esposito during the European Championships Champions Cup: in pain, Leinster beats Northampton and qualifies for the final
Champions Cup: in pain, Leinster beats Northampton and qualifies for the final Liga: Real Madrid crowned champion of Spain after FC Barcelona's defeat in Girona
Liga: Real Madrid crowned champion of Spain after FC Barcelona's defeat in Girona