A Japanese pharmaceutical group said Tuesday it was investigating one death and having received reports of several dozen hospitalizations, these cases being potentially linked to food supplements for which it had announced a recall last week. Kobayashi Pharmaceutical, a company based in Osaka (west), had already recognized on Monday that the hospitalization of 26 people was possibly linked to some of its food supplements, sold without a prescription.
Now the company has received reports of 50 additional hospitalizations as of Tuesday morning, but which are not yet confirmed at this stage, a spokesperson for the group told AFP. All three product lines recalled by Kobayashi Pharmaceutical contain a type of yeast-fermented rice called “beni koji” or red yeast rice, which is believed to be a cholesterol-lowering agent.
The group specified that it had supplied this yeast to around fifty other companies in Japan and two in Taiwan. “We are aware of one case with a potential causal link between a death and our product,” Kobayashi Pharmaceutical said in a statement. “We are currently investigating this link and what happened,” added the group, which offered its “deepest apologies”.
The deceased had regularly purchased one of the recalled products for nearly three years, according to the manufacturer. Japanese Health Minister Keizo Takemi told reporters on Tuesday that his ministry had asked Kobayashi Pharmaceutical to quickly provide more information. The ministry “will cooperate with the city of Osaka to investigate the cause (of the case, Editor’s note) and prevent further health damage,” assured Keizo Takemi.
The recall of three product lines, announced Friday by Kobayashi Pharmaceutical, followed customer complaints about kidney problems. The group said Monday that no conclusions had been drawn as to a possible cause and effect link between its food supplements and hospitalizations. “After analysis, we found that it was possible that the raw materials used to make beni koji contained ingredients that our company did not intend to include,” the group admitted, however.
According to him, his analyzes did not reveal the presence of citrinin, a toxic substance which can develop in red yeast rice and which can cause kidney problems. Local media reported that Kobayashi Pharmaceutical officials would visit the deceased's family on Tuesday.

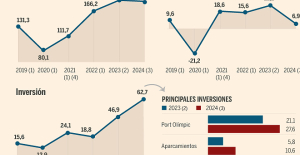 B:SM will break its investment record this year with 62 million euros
B:SM will break its investment record this year with 62 million euros War in Ukraine: when kyiv attacks Russia with inflatable balloons loaded with explosives
War in Ukraine: when kyiv attacks Russia with inflatable balloons loaded with explosives United States: divided on the question of presidential immunity, the Supreme Court offers respite to Trump
United States: divided on the question of presidential immunity, the Supreme Court offers respite to Trump Maurizio Molinari: “the Scurati affair, a European injury”
Maurizio Molinari: “the Scurati affair, a European injury” First three cases of “native” cholera confirmed in Mayotte
First three cases of “native” cholera confirmed in Mayotte Meningitis: compulsory vaccination for babies will be extended in 2025
Meningitis: compulsory vaccination for babies will be extended in 2025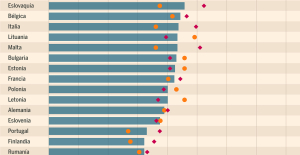 Spain is the country in the European Union with the most overqualified workers for their jobs
Spain is the country in the European Union with the most overqualified workers for their jobs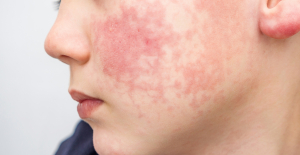 Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024
Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024 Inflation rebounds in March in the United States, a few days before the Fed meeting
Inflation rebounds in March in the United States, a few days before the Fed meeting Video games: Blizzard cancels Blizzcon 2024, its annual high mass
Video games: Blizzard cancels Blizzcon 2024, its annual high mass Falling wings of the Moulin Rouge: who will pay for the repairs?
Falling wings of the Moulin Rouge: who will pay for the repairs? “You don’t sell a company like that”: Roland Lescure “annoyed” by the prospect of a sale of Biogaran
“You don’t sell a company like that”: Roland Lescure “annoyed” by the prospect of a sale of Biogaran Exhibition: in Deauville, Zao Wou-Ki, beauty in all things
Exhibition: in Deauville, Zao Wou-Ki, beauty in all things Dak’art, the most important biennial of African art, postponed due to lack of funding
Dak’art, the most important biennial of African art, postponed due to lack of funding In Deadpool and Wolverine, Ryan and Hugh Jackman explore the depths of the Marvel multiverse
In Deadpool and Wolverine, Ryan and Hugh Jackman explore the depths of the Marvel multiverse Tom Cruise returns to Paris for the filming of Mission Impossible 8
Tom Cruise returns to Paris for the filming of Mission Impossible 8 Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars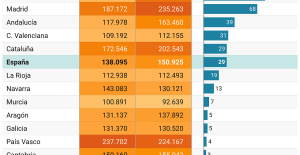 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade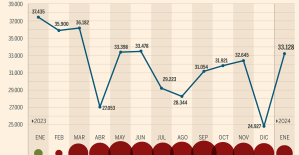 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Even on a mission for NATO, the Charles-de-Gaulle remains under French control, Lecornu responds to Mélenchon
Even on a mission for NATO, the Charles-de-Gaulle remains under French control, Lecornu responds to Mélenchon “Deadly Europe”, “economic decline”, immigration… What to remember from Emmanuel Macron’s speech at the Sorbonne
“Deadly Europe”, “economic decline”, immigration… What to remember from Emmanuel Macron’s speech at the Sorbonne Sale of Biogaran: The Republicans write to Emmanuel Macron
Sale of Biogaran: The Republicans write to Emmanuel Macron Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Euroleague: at the end of the suspense, Monaco equalizes against Fenerbahçe
Euroleague: at the end of the suspense, Monaco equalizes against Fenerbahçe Women's Six Nations: Where to see and five things to know about France-England
Women's Six Nations: Where to see and five things to know about France-England Liverpool: it is confirmed, Slot will succeed Klopp on the Reds bench
Liverpool: it is confirmed, Slot will succeed Klopp on the Reds bench Ligue 1: Montpellier and Nantes back to back, two reds in stoppage time
Ligue 1: Montpellier and Nantes back to back, two reds in stoppage time