Wednesday's posting by the Food and Drug Administration included much of what its advisory panel will be considering. The agency took a neutral tone regarding boosters, which is unusual considering that President Joe Biden and his top advisors had announced that they would be starting a booster campaign next week.
Pfizer's argument is that while severe diseases are still protected in the United States, immunity to milder infections decreases around six to eight month after the second dose. At that point, the company gave 306 more doses to patients and found levels of virus-fighting antibody three times higher than those who received the first shots.
Pfizer stated that the antibodies are strong enough to fight the extra-contagious Delta variant spreading around the country.
Pfizer provided data from Israel to support its case. The FDA began offering boosters in the summer.
The study involved 1,000,000 people aged 60 or older. It found that those who received the additional shot were less likely to contract the disease soon afterward. Pfizer stated that this translates into "roughly 95% effectiveness" as delta spreads, which is comparable to protection received shortly after the vaccine was introduced earlier in the year.
Data from Israel, published Wednesday in The New England Journal of Medicine, does not indicate how long the boosted protection will last.
However, FDA reviewers suggested that they would focus on research on vaccine effectiveness among Americans.
The data indicate that Pfizer and other U.S.-authorized COVID-19 vaccinations "still provide protection against severe COVID-19 diseases and death in the United States," according to the agency.
The FDA isn't bound to follow the advice from its independent advisory panel. The FDA could confuse the public if it ignores its independent advisory panel. Two top FDA vaccine regulators joined an international group of scientists in rejecting boosters for otherwise healthy people earlier this week. They cited the strong ongoing protection against severe diseases.

 War in Ukraine: when kyiv attacks Russia with inflatable balloons loaded with explosives
War in Ukraine: when kyiv attacks Russia with inflatable balloons loaded with explosives United States: divided on the question of presidential immunity, the Supreme Court offers respite to Trump
United States: divided on the question of presidential immunity, the Supreme Court offers respite to Trump Maurizio Molinari: “the Scurati affair, a European injury”
Maurizio Molinari: “the Scurati affair, a European injury” Hamas-Israel war: US begins construction of pier in Gaza
Hamas-Israel war: US begins construction of pier in Gaza First three cases of “native” cholera confirmed in Mayotte
First three cases of “native” cholera confirmed in Mayotte Meningitis: compulsory vaccination for babies will be extended in 2025
Meningitis: compulsory vaccination for babies will be extended in 2025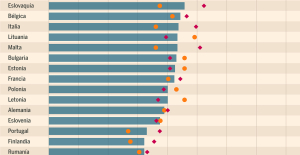 Spain is the country in the European Union with the most overqualified workers for their jobs
Spain is the country in the European Union with the most overqualified workers for their jobs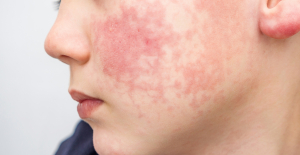 Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024
Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024 Inflation rebounds in March in the United States, a few days before the Fed meeting
Inflation rebounds in March in the United States, a few days before the Fed meeting Video games: Blizzard cancels Blizzcon 2024, its annual high mass
Video games: Blizzard cancels Blizzcon 2024, its annual high mass Falling wings of the Moulin Rouge: who will pay for the repairs?
Falling wings of the Moulin Rouge: who will pay for the repairs? “You don’t sell a company like that”: Roland Lescure “annoyed” by the prospect of a sale of Biogaran
“You don’t sell a company like that”: Roland Lescure “annoyed” by the prospect of a sale of Biogaran Exhibition: in Deauville, Zao Wou-Ki, beauty in all things
Exhibition: in Deauville, Zao Wou-Ki, beauty in all things Dak’art, the most important biennial of African art, postponed due to lack of funding
Dak’art, the most important biennial of African art, postponed due to lack of funding In Deadpool and Wolverine, Ryan and Hugh Jackman explore the depths of the Marvel multiverse
In Deadpool and Wolverine, Ryan and Hugh Jackman explore the depths of the Marvel multiverse Tom Cruise returns to Paris for the filming of Mission Impossible 8
Tom Cruise returns to Paris for the filming of Mission Impossible 8 Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars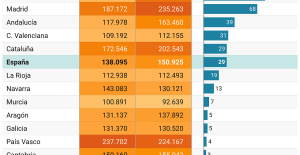 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade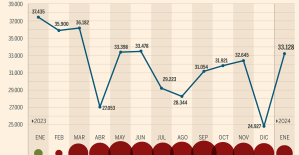 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella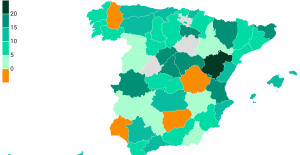 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Even on a mission for NATO, the Charles-de-Gaulle remains under French control, Lecornu responds to Mélenchon
Even on a mission for NATO, the Charles-de-Gaulle remains under French control, Lecornu responds to Mélenchon “Deadly Europe”, “economic decline”, immigration… What to remember from Emmanuel Macron’s speech at the Sorbonne
“Deadly Europe”, “economic decline”, immigration… What to remember from Emmanuel Macron’s speech at the Sorbonne Sale of Biogaran: The Republicans write to Emmanuel Macron
Sale of Biogaran: The Republicans write to Emmanuel Macron Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Basketball: Strasbourg appeals the victory recovered by Monaco
Basketball: Strasbourg appeals the victory recovered by Monaco Top 14: UBB with Tatafu and Moefana against Bayonne
Top 14: UBB with Tatafu and Moefana against Bayonne MotoGP: Bagnaia dominates qualifying practice in Spain and sets track record
MotoGP: Bagnaia dominates qualifying practice in Spain and sets track record Olympic Games: in Athens, Greece transmits the Olympic flame to France
Olympic Games: in Athens, Greece transmits the Olympic flame to France