To reduce the diagnostic wandering experienced by many women with endometriosis, the High Authority for Health is opening the door to the coverage of a saliva test that it considers “promising”, but is awaiting new data before a possible general reimbursement.
The test, called Endotest and developed by the Lyon biotech Ziwig, “showed very good diagnostic performance,” underlines the HAS, which took action to evaluate its effectiveness and usefulness. “It’s a clear and strong recognition of our work, it’s extremely positive,” Yahya El Mir, the founder and president of Ziwig, told AFP.
Also read: Endometriosis: “Doctors talk about fertility to women who have much more urgent pain”
Endometriosis is a chronic disease affecting around one in ten women. It usually results in severe pain during periods and/or fertility problems. Even today, it is diagnosed, often by chance, with an average delay of seven years. Reducing this delay to a few days thanks to a saliva test intended for symptomatic women is nothing other than a “revolution”, praises the founder of the start-up.
How does this test work? “It involves taking a little saliva, which contains micro-RNAs,” explains Yahya El Mir. Thanks to saliva sampling, it is possible “to get as close as possible to the biological functioning of cells and produce information that is not obtained through imaging or surgery, and which allows a biological diagnosis to be made. safe,” says Mr. El Mir. The test, which aims to avoid a potentially invasive laparoscopy, then involves carrying out high-throughput sequencing and the use of an algorithm designed by artificial intelligence.
“I wanted to rip my stomach out”: endometriosis, a scourge for mental health
A year ago, Inserm (National Institute of Health and Medical Research) remained cautious about the results of a first study including only 200 patients. The High Health Authority issued its opinion on Monday based on the extension of this same study to more than 1,000 women suffering from pelvic pain. Her evaluation highlighted a diagnostic accuracy of 95% for this test which she considers “promising” and “innovative”.
Although it recognizes “high expectations” from patients for this test, the HAS nevertheless emphasizes “the need to carry out additional studies aimed at evaluating its clinical usefulness in current practice”. Consequently, it initially offers early access, via a so-called “innovation” package. This allows, specifies the HAS on its website, “exempt and temporary support to facilitate early access for patients to innovative technologies (health technologies other than medicines) in the early phase of clinical development”. Concretely, if the advice of the HAS is followed by the government, women over 18 years of age, for whom endometriosis is “strongly suspected”, will be able to carry out this test free of charge. Support, however, is “conditional” on participation in new studies, which will make it possible to decide whether or not in favor of long-term reimbursement. “We are particularly waiting to know if this test will make it possible to improve the treatment strategy,” we explain to the HAS. “We will be careful to ensure that this does not delay access to our test for women in need,” assures the founder of Ziwig.
For patients, the marketing and reimbursement of the test could “be a game changer,” Priscilla Saracco, general director of the Endomind association, told AFP. “It is incomprehensible not to quickly take all the necessary measures to make it widely accessible,” the association reacted in a press release on Monday. “The refusal to provide rapid reimbursement for the saliva test, a global innovation which would allow thousands of women to finally obtain answers, disregards the interests of patients,” according to her. The Endotest has been sold for more than a year in around ten countries in Europe and the Middle East, “at around 1,000 euros”, according to Ziwig.
However, “there is no technique more precise than this test,” says Hervé Fernandez, gynecological surgeon, professor emeritus at Paris Saclay University. “But we have to ask ourselves what we are going to do with our results, what treatments we will then be able to offer.” Today, there is no definitive treatment for endometriosis, although hormonal therapy and/or surgery can sometimes stem its progression.
Ziwig is working on a second version of the test which will be able to specify the characteristics of the disease depending on the patient (superficial form of endometriosis, increased risk of infertility, etc.), to adjust treatments.

 Finding yourself face to face with a man or a bear? The debate that shakes up social networks
Finding yourself face to face with a man or a bear? The debate that shakes up social networks Sabadell rejects the merger with BBVA and will fight to remain alone
Sabadell rejects the merger with BBVA and will fight to remain alone In Germany, the far left wants to cap the price of “doner kebabs”
In Germany, the far left wants to cap the price of “doner kebabs” Israel-Hamas war: Gaza between hope of truce and fear of Israeli offensive in the South
Israel-Hamas war: Gaza between hope of truce and fear of Israeli offensive in the South The presence of blood in the urine, a warning sign of bladder cancer
The presence of blood in the urine, a warning sign of bladder cancer A baby whose mother smoked during pregnancy will age more quickly
A baby whose mother smoked during pregnancy will age more quickly The euro zone economy grows in April at its best pace in almost a year but inflationary pressure increases
The euro zone economy grows in April at its best pace in almost a year but inflationary pressure increases Children born thanks to PMA do not have more cancers than others
Children born thanks to PMA do not have more cancers than others “The prices are astronomical”: to enjoy the Olympics with family, Sabrina spent... 8,000 euros
“The prices are astronomical”: to enjoy the Olympics with family, Sabrina spent... 8,000 euros “House of the Dragon”, “Succession”… Max, the new streaming platform from HBO and Discovery, launched in France on June 11
“House of the Dragon”, “Succession”… Max, the new streaming platform from HBO and Discovery, launched in France on June 11 The A13 motorway will finally reopen this Friday, in one direction only
The A13 motorway will finally reopen this Friday, in one direction only TNT commission of inquiry: tensions between LFI deputies and Macronists before the vote on the report
TNT commission of inquiry: tensions between LFI deputies and Macronists before the vote on the report The Coubertin Spirit, Eternal Memory, Planet of the Apes... Films to watch this week
The Coubertin Spirit, Eternal Memory, Planet of the Apes... Films to watch this week Rappers Drake and Kendrick Lamar vie for the spotlight
Rappers Drake and Kendrick Lamar vie for the spotlight In Istanbul, the ancient Byzantine church of Saint-Sauveur-in-Chora reopens for Muslim worship
In Istanbul, the ancient Byzantine church of Saint-Sauveur-in-Chora reopens for Muslim worship David Castello-Lopes: a fresh wind blows on the one-man show with his show Authentique
David Castello-Lopes: a fresh wind blows on the one-man show with his show Authentique Omoda 7, another Chinese car that could be manufactured in Spain
Omoda 7, another Chinese car that could be manufactured in Spain BYD chooses CA Auto Bank as financial partner in Spain
BYD chooses CA Auto Bank as financial partner in Spain Tesla and Baidu sign key agreement to boost development of autonomous driving
Tesla and Baidu sign key agreement to boost development of autonomous driving Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV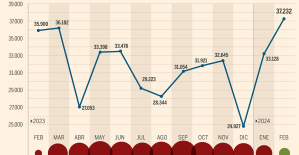 The home mortgage firm rises 3.8% in February and the average interest moderates to 3.33%
The home mortgage firm rises 3.8% in February and the average interest moderates to 3.33%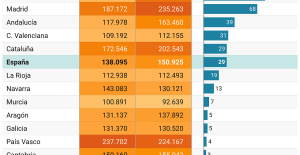 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade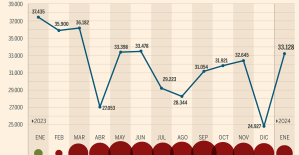 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella Institutions: senators want to restore the accumulation of mandates and put an end to the automatic presence of ex-presidents on the Constitutional Council
Institutions: senators want to restore the accumulation of mandates and put an end to the automatic presence of ex-presidents on the Constitutional Council Europeans: David Lisnard expresses his “essential and vital” support for François-Xavier Bellamy
Europeans: David Lisnard expresses his “essential and vital” support for François-Xavier Bellamy Facing Jordan Bardella, the popularity match turns to Gabriel Attal’s advantage
Facing Jordan Bardella, the popularity match turns to Gabriel Attal’s advantage Europeans: a senior official on the National Rally list
Europeans: a senior official on the National Rally list These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Ligue 1: at what time and on which channel to watch the Multiplex on Sunday?
Ligue 1: at what time and on which channel to watch the Multiplex on Sunday? PSG-Dortmund: Hummels’ (big) tackle to “teams who wanted to play against” BVB
PSG-Dortmund: Hummels’ (big) tackle to “teams who wanted to play against” BVB Top 14: Pierre Bochaton extends with UBB until 2027
Top 14: Pierre Bochaton extends with UBB until 2027 Tennis: Monfils beaten, Atmane surprises and advances to the 2nd round in Rome
Tennis: Monfils beaten, Atmane surprises and advances to the 2nd round in Rome