They manipulate their host's cells, defend themselves against predators or kill off competitors: many bacteria use sophisticated molecular nanosyringes to inject proteins into cells. These so-called contractile injection systems (CIS) can be reprogrammed and may be used therapeutically in many different ways in the future, as US researchers report in the journal Nature.
The team led by Feng Zhang from the Broad Institute in Cambridge (Massachusetts, USA) modified a bacterial nanosyringe in such a way that it could target active substances to specific cell types. The group writes that this could make various therapies possible, referring in particular to cancer treatments and gene therapies.
Clemens Wendtner from the Munich Clinic Schwabing speaks of a "revolutionary technology". "It seems that we are on the threshold of a new development," comments the doctor, who was not involved in the study. "Here there are no limits to the imagination with regard to future applications." Other experts also see a breakthrough in the study that could open up many options.
In the proof of concept, Zhang's team studied the injection system of the bacterium Photorhabdus asymbiotica, which originally targets insect cells. The molecular syringe - called the Photorhabdus virulence cassette (PVC) - consists of a tube about 100 nanometers (millionths of a millimeter) long. At its end, a so-called tail fiber binds to special receptors on the target cells so that the protein load can be guided through the cell membrane into these cells.
In systematic sub-tests, Zhang's team changed the injection devices in two main ways: On the one hand, they were able to initially inject other proteins that do not originate from P. asymbiotica into insect cells. Secondly, it reprogrammed the tail fibers in such a way that the nanosyringes attached to other cells, for example those of mice or humans.
For example, the researchers made sure that the injection system in the laboratory docked onto cells from lung tumors and killed them with a toxin. In another experiment, they introduced the Cas9 enzyme – the component of the Crispr-Cas9 gene scissors that can cut DNA – into human cells. This could potentially make it possible in the future to therapeutically modify the DNA in cells at desired targets.
In a final step, the team demonstrated the use of the nanosyringe on living organisms. By injecting them into the brains of mice, they smuggled proteins into nerve cells in the brain area of the hippocampus. The researchers observed neither cell-damaging effects nor a strong activation of the immune system.
In addition, the injection apparatus was no longer detectable after one week. "This suggests that the system is ideally suited for therapies intended to be transient or short-term," the group notes.
The system presented makes it possible to "inject any proteins into cells with any defined structures on their surface," says Andreas Diepold from the Max Planck Institute for Terrestrial Microbiology in Marburg. Such an injection system, which can be loaded with foreign proteins, is a breakthrough.
However, experts point to a few hurdles: the protein load in the system is limited. In addition, it is important that this charge is only brought to the desired destinations and does not reach any other cells.
"The ability to introduce specific proteins into specific cell types would offer tremendous potential both for research in the life sciences and for the treatment of diseases," write Charles Ericson and Martin Pilhofer from the Swiss Federal Institute of Technology in Zurich (ETH). a "Nature" comment. "These transformed injection complexes represent an exciting biotechnological toolbox with applications in diverse biological systems."
Study leader Feng Zhang is a prominent figure in the life sciences. For the development of the Crispr-Cas9 gene scissors, he fought a bitter patent dispute with the two researchers Emmanuelle Charpentier, now founding director of the Max Planck Research Center for the Science of Pathogens, and Jennifer Doudna from the University of California at Berkeley.
They presented their work on the method in quick succession in 2012 in Science magazine. Charpentier and Doudna received the Nobel Prize for Medicine in 2020, Zhang went away empty-handed.

 Hamas-Israel war: US begins construction of pier in Gaza
Hamas-Israel war: US begins construction of pier in Gaza Israel prepares to attack Rafah
Israel prepares to attack Rafah Indifference in European capitals, after Emmanuel Macron's speech at the Sorbonne
Indifference in European capitals, after Emmanuel Macron's speech at the Sorbonne Spain: what is Manos Limpias, the pseudo-union which denounced the wife of Pedro Sánchez?
Spain: what is Manos Limpias, the pseudo-union which denounced the wife of Pedro Sánchez?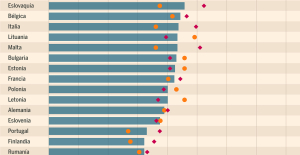 Spain is the country in the European Union with the most overqualified workers for their jobs
Spain is the country in the European Union with the most overqualified workers for their jobs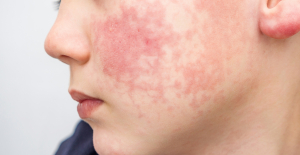 Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024
Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024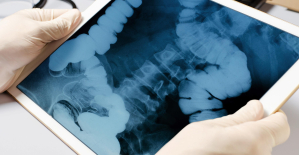 Colorectal cancer: what to watch out for in those under 50
Colorectal cancer: what to watch out for in those under 50 H5N1 virus: traces detected in pasteurized milk in the United States
H5N1 virus: traces detected in pasteurized milk in the United States Private clinics announce a strike with “total suspension” of their activities, including emergencies, from June 3 to 5
Private clinics announce a strike with “total suspension” of their activities, including emergencies, from June 3 to 5 The Lagardère group wants to accentuate “synergies” with Vivendi, its new owner
The Lagardère group wants to accentuate “synergies” with Vivendi, its new owner The iconic tennis video game “Top Spin” returns after 13 years of absence
The iconic tennis video game “Top Spin” returns after 13 years of absence Three Stellantis automobile factories shut down due to supplier strike
Three Stellantis automobile factories shut down due to supplier strike A pre-Roman necropolis discovered in Italy during archaeological excavations
A pre-Roman necropolis discovered in Italy during archaeological excavations Searches in Guadeloupe for an investigation into the memorial dedicated to the history of slavery
Searches in Guadeloupe for an investigation into the memorial dedicated to the history of slavery Aya Nakamura in Olympic form a few hours before the Flames ceremony
Aya Nakamura in Olympic form a few hours before the Flames ceremony Psychiatrist Raphaël Gaillard elected to the French Academy
Psychiatrist Raphaël Gaillard elected to the French Academy Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars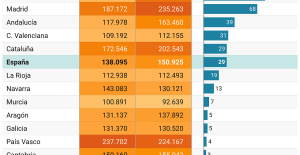 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade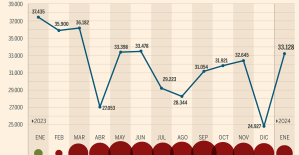 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella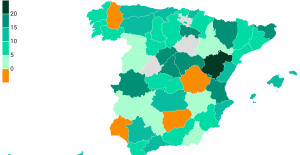 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? “Deadly Europe”, “economic decline”, immigration… What to remember from Emmanuel Macron’s speech at the Sorbonne
“Deadly Europe”, “economic decline”, immigration… What to remember from Emmanuel Macron’s speech at the Sorbonne Sale of Biogaran: The Republicans write to Emmanuel Macron
Sale of Biogaran: The Republicans write to Emmanuel Macron Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition
With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Judo: Blandine Pont European vice-champion
Judo: Blandine Pont European vice-champion Swimming: World Anti-Doping Agency appoints independent prosecutor in Chinese doping case
Swimming: World Anti-Doping Agency appoints independent prosecutor in Chinese doping case Water polo: everything you need to know about this sport
Water polo: everything you need to know about this sport Judo: Cédric Revol on the 3rd step of the European podium
Judo: Cédric Revol on the 3rd step of the European podium


















