Many major medical centers are still undecided about whether or not to use Biogen’s Aduhelm. This is the recommended treatment for the early stages of the disease. Major medical centers like Mass General Brigham in Boston and the Cleveland Clinic have said they will not use it.
One neurology practice even expelled the company's sales representatives from its offices due to concerns about the drug's price and potential for it to rise past $50,000 per year.
Many doctors feel they must learn more about Aduhelm and the benefits before they offer it to patients. This could take many months. There may be questions that remain even after the process is completed.
Salim Syed, an analyst covering Biogen for Mizuho Securities USA, stated that "the drug won't work for everyone, even with access."
Syed estimates that around one-tenth (or less) of people with early-stage Alzheimer’s will end up taking Aduhelm regularly, particularly if regulators approve similar treatments by Biogen's rivals.
Biogen reports its third quarter financial results Wednesday. However, it doesn't say how many people have been given the drug since June 7. A company executive stated last month that Biogen knew of approximately 50 Aduhelm sites, which is far less than the 900 it had claimed to have in its possession shortly after the drug was approved by regulators.
Aduhelm is one of a number of new drugs that promises to slow down the progression of this fatal brain-destroying disease.
Dr. Stephen Salloway of Rhode Island, a neurologist and Biogen consultant, said that the drug is "like a breath-taking fresh air." Alzheimer's patients "know what's ahead, and they want everything they can do to stay in the milder stages."
Aduhelm was approved by the U.S. Food and Drug Administration despite objections from several independent advisors who have since resigned. Later, the agency stated that Aduhelm was suitable for patients with mild cognitive impairments or early-stage Alzheimer’s.
Aduhelm removes brain plaque believed to be linked to Alzheimer's disease. The decision was made by regulators based on research results that showed the drug would likely benefit patients.
Biogen, which co-developed Aduhelm with Japan’s Eisai Co. had to stop two studies due to disappointing results. Later, it said that further analysis had shown the treatment's effectiveness at higher doses.
Biogen is required by the FDA to perform a follow up study.

 An American tourist steals a mug of beer from a tavern in Munich and admits it...50 years later
An American tourist steals a mug of beer from a tavern in Munich and admits it...50 years later Madagascar calls for replacement of EU ambassador
Madagascar calls for replacement of EU ambassador Chinese journalist released from prison four years after denouncing China's handling of Covid
Chinese journalist released from prison four years after denouncing China's handling of Covid “Russian law” adopted in Georgia: Europeans alongside the streets
“Russian law” adopted in Georgia: Europeans alongside the streets Suicide attempts and self-harm on the rise among young girls
Suicide attempts and self-harm on the rise among young girls In Europe, 10,000 people die every day from cardiovascular disease
In Europe, 10,000 people die every day from cardiovascular disease Brussels raises Spanish GDP forecasts to 2.1% and foresees a deficit of 3% in 2024
Brussels raises Spanish GDP forecasts to 2.1% and foresees a deficit of 3% in 2024 Inflation rises to 3.3% in April due to gas and food
Inflation rises to 3.3% in April due to gas and food Parisian owners of electric SUVs will have to pay a residential parking rate from October 1
Parisian owners of electric SUVs will have to pay a residential parking rate from October 1 Saint-Denis-Pleyel station, “a little Châtelet north of Paris”, ready to open before the Olympic Games
Saint-Denis-Pleyel station, “a little Châtelet north of Paris”, ready to open before the Olympic Games Comedian Guillaume Meurice, still “banned from the air”, awaits his possible sanction
Comedian Guillaume Meurice, still “banned from the air”, awaits his possible sanction GTA 6: the event video game will be released in fall 2025
GTA 6: the event video game will be released in fall 2025 The Le Bon Air festival deprograms the DJ I Hate Models for a private jet story
The Le Bon Air festival deprograms the DJ I Hate Models for a private jet story A golden reward for Yasmina Reza
A golden reward for Yasmina Reza Cannes: a demonstration of the precarious of cinema above the red carpet
Cannes: a demonstration of the precarious of cinema above the red carpet Magician David Copperfield accused of sexual violence by sixteen women
Magician David Copperfield accused of sexual violence by sixteen women Omoda 7, another Chinese car that could be manufactured in Spain
Omoda 7, another Chinese car that could be manufactured in Spain BYD chooses CA Auto Bank as financial partner in Spain
BYD chooses CA Auto Bank as financial partner in Spain Tesla and Baidu sign key agreement to boost development of autonomous driving
Tesla and Baidu sign key agreement to boost development of autonomous driving Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV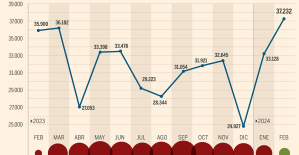 The home mortgage firm rises 3.8% in February and the average interest moderates to 3.33%
The home mortgage firm rises 3.8% in February and the average interest moderates to 3.33%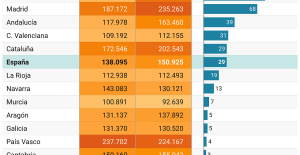 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade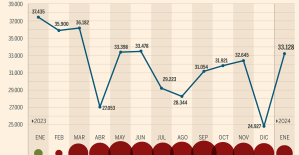 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella Europeans: between the RN and the “euro-gagas”, the communist Léon Deffontaines dreams of breaking through to the left
Europeans: between the RN and the “euro-gagas”, the communist Léon Deffontaines dreams of breaking through to the left Europeans: professor threatened with death Didier Lemaire shows his support for François-Xavier Bellamy
Europeans: professor threatened with death Didier Lemaire shows his support for François-Xavier Bellamy LFI: Manon Aubry places social issues at the center of her European campaign
LFI: Manon Aubry places social issues at the center of her European campaign Diving into the secrets of the National Assembly
Diving into the secrets of the National Assembly These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Athletics: Mélina Robert-Michon improves her best mark of the season at the Montreuil meeting
Athletics: Mélina Robert-Michon improves her best mark of the season at the Montreuil meeting Calendar, Mbappé in Madrid, Milanese in Australia: what awaits the Blues between now and the Euro
Calendar, Mbappé in Madrid, Milanese in Australia: what awaits the Blues between now and the Euro French team: Warren Zaire-Emery forced to postpone his baccalaureate... to participate in the Euro
French team: Warren Zaire-Emery forced to postpone his baccalaureate... to participate in the Euro French team: “The situation in my head was clear”, Deschamps explains Kanté’s return
French team: “The situation in my head was clear”, Deschamps explains Kanté’s return


















