On the occasion of International Rare Disease Day, Catherine Vautrin, Minister of Labor, Health and Solidarity, is expected to announce on Thursday February 29 the first elements of a fourth rare disease plan, eagerly awaited by patients and rare disease associations. sick. In an interview with Le Figaro, Hélène Berrué-Gaillard, the president of the Rare Disease Alliance, recalls that much remains to be done to ensure true equity in access to treatments and diagnosis for the 3 million French people affected by these pathologies. and care.
Le Figaro. - The fourth rare disease plan should see the light of day in the coming months. What assessment do you draw from the previous ones?
Hélène BERRUÉ-GAILLARD. - Supported by associations, and in particular by the AFM, the three plans made it possible to understand the public health issue of rare diseases which affect 3 million French people. One in 20 people is affected by one of the 6,300 pathologies known to date. France was the first country in Europe, or even in the world, to set up an identified care pathway with 23 sectors, 19 platforms and 2,311 referring hospital services. But today we are in the middle of the ford. In fact, only seven out of ten patients see a diagnosis made based on their symptoms. For 30% of them, the genetic analyzes carried out did not find any identified mutation. You should know that even today, rare diseases are unfortunately the leading cause of mortality in children. Three-quarters of them begin in childhood and 30% of affected children will not reach the age of 5! Even though there has been progress made, there are still delays in diagnosis and access to care. France has been a pioneer in the field of rare diseases, and it must remain so.
Also read: “Only 10% of rare diseases have dedicated treatment”
How can the fourth plan remedy these diagnostic delays?
Doctors are poorly trained and do not think enough about rare diseases. During an intervention with interns at the end of the course, I asked them what the number of people affected was in France. They told me 100,000. There are 30 times more! We must collectively spread the culture of doubt, summed up in a simple question: “What if it was a rare disease?” » Health professionals must ask themselves this question, but also the general public. As patients, parents of patients, we must be proactive in the face of the second cause of illness in France and the first in children. To do this, we call on public authorities to launch campaigns to raise awareness among the general public and train health professionals on the subject. Thus, RDK is a new application intended for health professionals, co-developed by Orphanet, which allows them, based on an entry by symptoms, to identify a potential rare disease and to be referred to an expert center. This application must be made known to health professionals.
The diagnosis is made in particular by genetic screening. Should access be improved?
Indeed, despite the France genomics plan which has enabled progress, we need faster access to sequencing. We have testimonials from people who are still waiting for the results of their genetic analysis after 3 years! Relying on the private sector by regulating prescriptions, to avoid abuses, could solve this problem. For the 30% of people in diagnostic impasse, we should also be able to repeat this sequencing regularly. Thanks to the discovery of new genetic mutations, it would be possible to find the origin of a disease for certain patients. Indeed, the analyzed genome can be compared to a model genome which evolves according to the discovery of new genetic mutations.
Also read: Europe must act for the 30 million people affected by rare diseases
The rare diseases platform also warns about birth screening, citing “non-assistance” to people in danger. What do you mean ?
But it is! France, which was one of the first countries to implement neonatal screening in the 1970s, is now lagging far behind. It detects 13 diseases at birth, a far cry from its European neighbors who sometimes detect nearly 30 to 40. However, the absence of screening at birth is an unacceptable loss of opportunity, even though there are treatments available. that can save children's lives and significantly change their futures. Take the example of infantile spinal muscular atrophy for which there is gene therapy, which must be carried out quickly after birth. Between 2019 and 2023, around sixty children died due to lack of sufficiently early diagnosis. When you finally learn that your child has died even though if he had been detected early at birth he could have been saved, it is intolerable. Currently, the HAS recommendation processes and the implementation of screenings are far too long. Due to a lack of political will, we are not providing enough resources. We must turn the table on neonatal screening: from the moment there is a treatment, an ongoing clinical trial or appropriate care, screening must be systematized.
More and more rare diseases have treatments available, particularly with the rise of gene therapy. Isn’t that good news?
It is true that research is particularly active and is finding more and more drug candidates. Even if, I remind you, 95% of rare diseases have no treatment. However, the pharmaceutical industry's investments focus on diseases that offer commercial prospects and neglect the rarest diseases. A telling example is that of Crigler-Najjar disease, which affects 1 in 1 million births. These are children who live under lamps otherwise their bilirubin levels increase and damage their brain. AFM via Généthon has discovered a drug candidate. But today, they are missing 24 million to develop it and access the market. And the pharmaceutical industry is not interested. The public authorities must take control. This is why we want a public innovation fund to be created as part of the fourth rare disease plan.
Also read: Genome editing technology authorized to target a rare blood disease
Concerning medications, I would also like to alert you to other problems. That in particular of old molecules repositioned in a rare disease. When they are out of stock or discontinued, which is now common, patients have no alternative. The medicines agency must take up the subject to support patients. Concerning children, prescriptions are mainly off-label (prescriptions for indications which are not provided for in the marketing authorization of the drug, Editor's note) and it is the parents who are most often responsible for dividing them, with the risk of overdose or underdosage. We must secure these prescriptions via pediatric compounding, as other countries do.

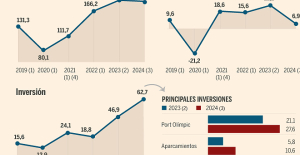 B:SM will break its investment record this year with 62 million euros
B:SM will break its investment record this year with 62 million euros War in Ukraine: when kyiv attacks Russia with inflatable balloons loaded with explosives
War in Ukraine: when kyiv attacks Russia with inflatable balloons loaded with explosives United States: divided on the question of presidential immunity, the Supreme Court offers respite to Trump
United States: divided on the question of presidential immunity, the Supreme Court offers respite to Trump Maurizio Molinari: “the Scurati affair, a European injury”
Maurizio Molinari: “the Scurati affair, a European injury” Beware of the three main sources of poisoning in children
Beware of the three main sources of poisoning in children First three cases of “native” cholera confirmed in Mayotte
First three cases of “native” cholera confirmed in Mayotte Meningitis: compulsory vaccination for babies will be extended in 2025
Meningitis: compulsory vaccination for babies will be extended in 2025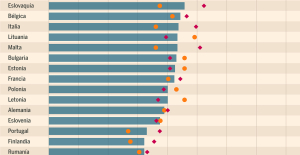 Spain is the country in the European Union with the most overqualified workers for their jobs
Spain is the country in the European Union with the most overqualified workers for their jobs In the United States, a Boeing 767 loses its emergency slide shortly after takeoff
In the United States, a Boeing 767 loses its emergency slide shortly after takeoff The A13 motorway will not reopen on May 1
The A13 motorway will not reopen on May 1 More than 1,500 items for less than 1 euro: the Dutch discounter Action opens a third store in Paris
More than 1,500 items for less than 1 euro: the Dutch discounter Action opens a third store in Paris 100 million euros in loans, water storage, Ecophyto plan… New measures from the executive towards farmers
100 million euros in loans, water storage, Ecophyto plan… New measures from the executive towards farmers New York justice returns 30 works of art looted from Cambodia and Indonesia
New York justice returns 30 works of art looted from Cambodia and Indonesia Les Galons de la BD dedicates War Photographers, a virtuoso album on the Spanish War
Les Galons de la BD dedicates War Photographers, a virtuoso album on the Spanish War Theater: Kevin, or the example of an academic failure
Theater: Kevin, or the example of an academic failure The eye of the INA: Jean Carmet, the thirst for life of a great actor
The eye of the INA: Jean Carmet, the thirst for life of a great actor Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars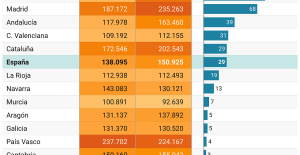 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade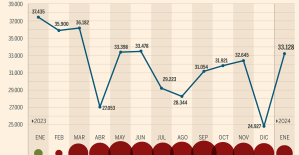 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella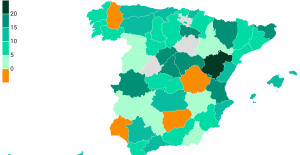 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Even on a mission for NATO, the Charles-de-Gaulle remains under French control, Lecornu responds to Mélenchon
Even on a mission for NATO, the Charles-de-Gaulle remains under French control, Lecornu responds to Mélenchon “Deadly Europe”, “economic decline”, immigration… What to remember from Emmanuel Macron’s speech at the Sorbonne
“Deadly Europe”, “economic decline”, immigration… What to remember from Emmanuel Macron’s speech at the Sorbonne Sale of Biogaran: The Republicans write to Emmanuel Macron
Sale of Biogaran: The Republicans write to Emmanuel Macron Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Tennis: “I need to regain confidence in my body,” explains Rafael Nadal
Tennis: “I need to regain confidence in my body,” explains Rafael Nadal NBA: Orlando returns to level with Cleveland in the 1st round of the play-offs
NBA: Orlando returns to level with Cleveland in the 1st round of the play-offs Tennis: Iga Swiatek in the round of 16 at full speed
Tennis: Iga Swiatek in the round of 16 at full speed “It was exceptional here in Chaban-Delmas”: Escudero looks back on the excitement around France-England
“It was exceptional here in Chaban-Delmas”: Escudero looks back on the excitement around France-England