now, a new Alzheimer's drug could be approved, on the level as the first active ingredient to the cause of the disease. On Thursday, Samantha presented to Budd by the U.S. pharmaceutical company Biogen an audience of specialists at the Alzheimer's disease conference (CTAD) in San Diego data from two large patient studies. The detailed analysis of the study results was long-awaited.
The two studies, each with approximately 1600 patients had made this year's headlines. They were stopped in March, because the substance, an antibody called Aducanumab seemed not to work. Then, Biogen announced that together with its Japanese Partner Eisai in October, in a re-analysis and further data, but an effect was found.
Now, independent experts were able to make yourself a picture and we are impressed. It was the first positive result of a drug against Alzheimer's disease for years, said the canadian Doctor Sharon Cohen. The were "very important results", was the neurologist Paul Aisen from San Diego. "I believe that the substance is," says Lutz Frölich Central Institute of Mental health in Mannheim, Germany. It has been convinced by the results, according to which it is clearly indicated that the effects are dose-dependent. That is, The patients in the study, the longest of the active substance in the highest dose, were the best.
changes in the brain
Add to that a small group of about 30 study participants was more closely examined. Imaging techniques showed in the brains of the Affected, that is exactly the went ill deposits in the brain are liable to return, to which the antibody attaches. Specifically, this means: In patients in an early stage of Alzheimer's disease, the progressive, developed at the end of Forget, thanks to Aducanumab slower than in the control group.
Also, the Biogen drug could stop Alzheimer's disease or missing mental skills to bring back. Nevertheless, If the Affected can work a year longer or your car independently, it was already a great success, says Sharon Cohen.
Biogen and Eisai had already announced in October, to apply for an authorisation for the active ingredient Aducanumab in the United States. If the competent authority, the FDA considers the data as sufficiently, could show up in a few months.
The other approach will probably look like that in the US study centers those patients who wish to re-Aducanumab be obtained. The Physicians in charge would collect additional data, which could later also be submitted to the approval authority. Frölich stresses that must prove the substance if it is allowed in practice. Then it will show whether Aducanumab works better than existing drugs. The side effects such as Nausea or headaches need to be followed.
it is Conceivable that a combination of the available drugs used for Alzheimer's therapy. And, of course, will also be the question of the cost. Frölich estimates that a new active ingredient that is administered once monthly as an Infusion, many times could be more expensive than treatment with the previously available resources.
Created: 09.12.2019, 09:11 PM

 The Euribor today remains at 3.734%
The Euribor today remains at 3.734% Germany: the trial of an AfD leader, accused of chanting a Nazi slogan, resumes this Tuesday
Germany: the trial of an AfD leader, accused of chanting a Nazi slogan, resumes this Tuesday New York: at Columbia University, the anti-Semitic drift of pro-Palestinian demonstrations
New York: at Columbia University, the anti-Semitic drift of pro-Palestinian demonstrations What is Akila, the mission in which the Charles de Gaulle is participating under NATO command?
What is Akila, the mission in which the Charles de Gaulle is participating under NATO command? What High Blood Pressure Does to Your Body (And Why It Should Be Treated)
What High Blood Pressure Does to Your Body (And Why It Should Be Treated) Vaccination in France has progressed in 2023, rejoices Public Health France
Vaccination in France has progressed in 2023, rejoices Public Health France Food additives suspected of promoting cardiovascular diseases
Food additives suspected of promoting cardiovascular diseases “Even morphine doesn’t work”: Léane, 17, victim of the adverse effects of an antibiotic
“Even morphine doesn’t work”: Léane, 17, victim of the adverse effects of an antibiotic MEPs validate reform of EU budgetary rules
MEPs validate reform of EU budgetary rules “Public Transport Paris 2024”, the application for Olympic Games spectators, is available
“Public Transport Paris 2024”, the application for Olympic Games spectators, is available Spotify goes green in the first quarter and sees its number of paying subscribers increase
Spotify goes green in the first quarter and sees its number of paying subscribers increase Xavier Niel finalizes the sale of his shares in the Le Monde group to an independent fund
Xavier Niel finalizes the sale of his shares in the Le Monde group to an independent fund Owner of Blondie and Shakira catalogs in favor of $1.5 billion offer
Owner of Blondie and Shakira catalogs in favor of $1.5 billion offer Cher et Ozzy Osbourne rejoignent le Rock and Roll Hall of Fame
Cher et Ozzy Osbourne rejoignent le Rock and Roll Hall of Fame Three months before the Olympic Games, festivals and concert halls fear paying the price
Three months before the Olympic Games, festivals and concert halls fear paying the price With Brigitte Macron, Aya Nakamura sows new clues about her participation in the Olympics
With Brigitte Macron, Aya Nakamura sows new clues about her participation in the Olympics Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars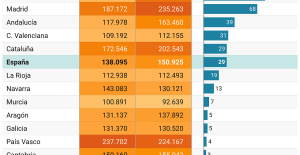 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade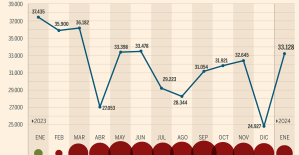 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella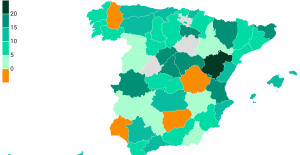 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition
With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition Europeans: the schedule of debates to follow between now and June 9
Europeans: the schedule of debates to follow between now and June 9 Europeans: “In France, there is a left and there is a right,” assures Bellamy
Europeans: “In France, there is a left and there is a right,” assures Bellamy These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Serie A: Bologna surprises AS Rome in the race for the C1
Serie A: Bologna surprises AS Rome in the race for the C1 Serie A: Marcus Thuram king of Italy, end of the debate for the position of number 9 with the Blues?
Serie A: Marcus Thuram king of Italy, end of the debate for the position of number 9 with the Blues? Milan AC-Inter Milan: Thuram and Pavard impeccable, Hernandez helpless… The tops and flops of the derby
Milan AC-Inter Milan: Thuram and Pavard impeccable, Hernandez helpless… The tops and flops of the derby Ligue 2: Auxerre leader, Bordeaux in crisis, play-offs... 5 questions about an exciting end of the season
Ligue 2: Auxerre leader, Bordeaux in crisis, play-offs... 5 questions about an exciting end of the season