Sounds almost like a christmas gift because it was a news long-awaited by physicians and patients that a short will have a chance to get out, too, from advanced melanoma. The Board of Directors of the Italian Agency of Drug (AIFA), in fact, has finally approved the redeemability of nivolumab, a drug immuno-oncology, in patients with melanoma in stage III and IV and completely resected. In the coming days is waiting for the publication in the Official Journal of the causes of the approval of the reimbursement. Why is it important adjuvant therapy For patients with advanced melanoma, the approval of the eligibility is really a breakthrough: “immuno-oncology has already demonstrated important results in metastatic, where it represents the standard of care – affirms Paolo Ascierto , director of the Oncology Unit of Melanoma, Immunotherapy of Cancer and Innovative Therapies of the National Cancer Institute, IRCCS Fondazione ‘Pascale’ of Naples. Today we have the possibility of early treatment in patients with stage III and IV resected, i.e. in a phase in which the disease has been completely removed. In patients with the disease (stage IIIB or IIIC, did not undergo adjuvant therapy after surgical resection, the recurrence rate at 5 years is high, at 71% and 85%. Treat patients in this stage increases the chances of avoiding a recurrence of the disease and, therefore, potentially to heal the person. In Italy, more than 1,000 patients each year may benefit from this treatment”.
Read also: "immuno-oncology is effective in the early stage" The data on the survival and on the risk of relapse In the study, CheckMate-238, nivolumab has demonstrated a long-term benefit in the setting of adjuvant with a relapse-free survival at three years of 58% and a reduction in the risk of recurrence of 32%. “The metastasis-free survival, distance - stresses Ascierto - is significantly longer with nivolumab, with rates at 36 months 66%. These benefits, which will have an impact on overall survival are observed in all patient subgroups analysed, regardless of the stage of disease, mutational status of the BRAF gene and the expression of PD-L1”. The perspective of the ‘end’ of therapy for 20 years, there had been steps forward in the adjuvant setting since the only two approved therapies, both of which are based on interferon, was associated with high toxicity and little is to be gained in terms of overall survival. The different perspectives offered by nivolumab, which guarantees, in addition to high efficacy, a good quality of life thanks to the good tolerability. “Adjuvant therapy with nivolumab continues, Ascierto - should be started possible after complete removal of the tumor and the treatment only lasts a year. The prospect of ‘the end’ of therapy represents a significant psychological advantage to the patients. These drugs have the capacity to develop a memory in the immune system, which keeps the ability to eliminate the cancer cells in the long term, even after discontinuation of therapy”. The reaction of the patients In 2019, in Italy, are estimated to be about 12,300 new cases of melanoma, the tumor tends to be more and more widespread even among young people. “I'm glad that, even in Italy, this treatment will be available for patients,” says Monica Fork , president APaIM (Association of Patients Italy Melanoma). “Also, for the people affected by the disease, it is important to know that this treatment has a duration of 12 months, representing a significant psychological advantage for patients is often young people who, in this way, you can deal with more strength in the course of treatment. In addition, the tolerability of the treatment, allows to maintain a good quality of life. Finally, not to be underestimated and the active involvement of the patient, who must be aware of the progress achieved by the research. Patients should be informed also about the ongoing trials: the inclusion in a clinical study, may allow earlier access to innovative therapies.” How does nivolumab Nivolumab is an inhibitor of checkpoint immune PD-1 (programmed death-1), which is able to restore and enhance the activity of our immune system against cancer cells. Our immune system is thus strengthened, in order to re-establish the immune response and anti-tumor. Nivolumab has become an important treatment option for many types of cancer. To date, more than 35,000 patients were enrolled in the program of clinical development of nivolumab in several cancers, with 15 publications in the prestigious scientific journal The New England Journal of Medicine, and eight phase III trials were terminated early because they had achieved survival benefit. The study CheckMate -238 led to the approval by the European Commission, in July 2018, the use of nivolumab in the adjuvant treatment of adult patients with melanoma with involvement of lymph nodes or metastatic disease, who underwent complete resection. Were involved more than 900 patients and the results were published in the prestigious scientific journal The New England Journal of Medicine. The initiatives of the expanded access waiting the arrival of the redeemability in Italy, the manufacturer of the drug, Bristol-Myers Squibb Italy, has started in the last 3 years of the programs expanded access (Expanded Access Program, EAP) by providing medications to more than 5,000 patients with various forms of cancer, in particular carcinoma of the lung, kidney, melanoma and Hodgkin's lymphoma. In this way, you are offered the possibility to access in a rapid effective therapies already approved in the United States and in Europe, before the conclusion of the process of redeemability in Italy. Even in the setting of adjuvant was started in a therapeutic program, ensuring that approximately 700 patients the opportunity to have free access to this treatment prior to eligibility for AIFA. In this program, the Institute Pascale was the first enroller in Italy with approximately 80 patients, proving once again as one of the reference centers for the treatment of melanoma in Italy.
Read also: "immuno-oncology is effective in the early stage" The data on the survival and on the risk of relapse In the study, CheckMate-238, nivolumab has demonstrated a long-term benefit in the setting of adjuvant with a relapse-free survival at three years of 58% and a reduction in the risk of recurrence of 32%. “The metastasis-free survival, distance - stresses Ascierto - is significantly longer with nivolumab, with rates at 36 months 66%. These benefits, which will have an impact on overall survival are observed in all patient subgroups analysed, regardless of the stage of disease, mutational status of the BRAF gene and the expression of PD-L1”. The perspective of the ‘end’ of therapy for 20 years, there had been steps forward in the adjuvant setting since the only two approved therapies, both of which are based on interferon, was associated with high toxicity and little is to be gained in terms of overall survival. The different perspectives offered by nivolumab, which guarantees, in addition to high efficacy, a good quality of life thanks to the good tolerability. “Adjuvant therapy with nivolumab continues, Ascierto - should be started possible after complete removal of the tumor and the treatment only lasts a year. The prospect of ‘the end’ of therapy represents a significant psychological advantage to the patients. These drugs have the capacity to develop a memory in the immune system, which keeps the ability to eliminate the cancer cells in the long term, even after discontinuation of therapy”. The reaction of the patients In 2019, in Italy, are estimated to be about 12,300 new cases of melanoma, the tumor tends to be more and more widespread even among young people. “I'm glad that, even in Italy, this treatment will be available for patients,” says Monica Fork , president APaIM (Association of Patients Italy Melanoma). “Also, for the people affected by the disease, it is important to know that this treatment has a duration of 12 months, representing a significant psychological advantage for patients is often young people who, in this way, you can deal with more strength in the course of treatment. In addition, the tolerability of the treatment, allows to maintain a good quality of life. Finally, not to be underestimated and the active involvement of the patient, who must be aware of the progress achieved by the research. Patients should be informed also about the ongoing trials: the inclusion in a clinical study, may allow earlier access to innovative therapies.” How does nivolumab Nivolumab is an inhibitor of checkpoint immune PD-1 (programmed death-1), which is able to restore and enhance the activity of our immune system against cancer cells. Our immune system is thus strengthened, in order to re-establish the immune response and anti-tumor. Nivolumab has become an important treatment option for many types of cancer. To date, more than 35,000 patients were enrolled in the program of clinical development of nivolumab in several cancers, with 15 publications in the prestigious scientific journal The New England Journal of Medicine, and eight phase III trials were terminated early because they had achieved survival benefit. The study CheckMate -238 led to the approval by the European Commission, in July 2018, the use of nivolumab in the adjuvant treatment of adult patients with melanoma with involvement of lymph nodes or metastatic disease, who underwent complete resection. Were involved more than 900 patients and the results were published in the prestigious scientific journal The New England Journal of Medicine. The initiatives of the expanded access waiting the arrival of the redeemability in Italy, the manufacturer of the drug, Bristol-Myers Squibb Italy, has started in the last 3 years of the programs expanded access (Expanded Access Program, EAP) by providing medications to more than 5,000 patients with various forms of cancer, in particular carcinoma of the lung, kidney, melanoma and Hodgkin's lymphoma. In this way, you are offered the possibility to access in a rapid effective therapies already approved in the United States and in Europe, before the conclusion of the process of redeemability in Italy. Even in the setting of adjuvant was started in a therapeutic program, ensuring that approximately 700 patients the opportunity to have free access to this treatment prior to eligibility for AIFA. In this program, the Institute Pascale was the first enroller in Italy with approximately 80 patients, proving once again as one of the reference centers for the treatment of melanoma in Italy.
"The Republic will fight always in defense of the freedom of information, to its readers and to all those who have at heart the principles of democracy and civil coexistence"
Carlo Verdelli SUBSCRIBERS TO REPUBLIC © Reproduction reserved Today on Gualtieri: "the presence of The State in the economy should not be taboo. It also serves to improve the functioning of the market" Government, now the Country deserves more in The line of the Highways don't Count. “Review of the concessions is the right of the government,” The company closes in the fort: “We are ready to defend ourselves” “Rates went up much more than inflation”the Republic

 Germany: Man armed with machete enters university library and threatens staff
Germany: Man armed with machete enters university library and threatens staff His body naturally produces alcohol, he is acquitted after a drunk driving conviction
His body naturally produces alcohol, he is acquitted after a drunk driving conviction Who is David Pecker, the first key witness in Donald Trump's trial?
Who is David Pecker, the first key witness in Donald Trump's trial? What does the law on the expulsion of migrants to Rwanda adopted by the British Parliament contain?
What does the law on the expulsion of migrants to Rwanda adopted by the British Parliament contain?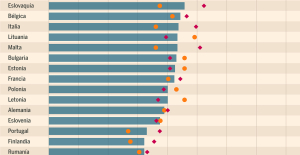 Spain is the country in the European Union with the most overqualified workers for their jobs
Spain is the country in the European Union with the most overqualified workers for their jobs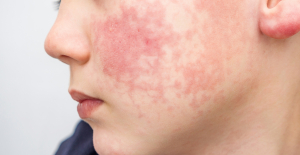 Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024
Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024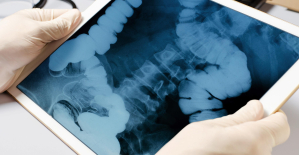 Colorectal cancer: what to watch out for in those under 50
Colorectal cancer: what to watch out for in those under 50 H5N1 virus: traces detected in pasteurized milk in the United States
H5N1 virus: traces detected in pasteurized milk in the United States Insurance: SFAM, subsidiary of Indexia, placed in compulsory liquidation
Insurance: SFAM, subsidiary of Indexia, placed in compulsory liquidation Under pressure from Brussels, TikTok deactivates the controversial mechanisms of its TikTok Lite application
Under pressure from Brussels, TikTok deactivates the controversial mechanisms of its TikTok Lite application “I can’t help but panic”: these passengers worried about incidents on Boeing
“I can’t help but panic”: these passengers worried about incidents on Boeing “I’m interested in knowing where the money that the State takes from me goes”: Bruno Le Maire’s strange pay slip sparks controversy
“I’m interested in knowing where the money that the State takes from me goes”: Bruno Le Maire’s strange pay slip sparks controversy 25 years later, the actors of Blair Witch Project are still demanding money to match the film's record profits
25 years later, the actors of Blair Witch Project are still demanding money to match the film's record profits At La Scala, Mathilde Charbonneaux is Madame M., Jacqueline Maillan
At La Scala, Mathilde Charbonneaux is Madame M., Jacqueline Maillan Deprived of Hollywood and Western music, Russia gives in to the charms of K-pop and manga
Deprived of Hollywood and Western music, Russia gives in to the charms of K-pop and manga Exhibition: Toni Grand, the incredible odyssey of a sculptural thinker
Exhibition: Toni Grand, the incredible odyssey of a sculptural thinker Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars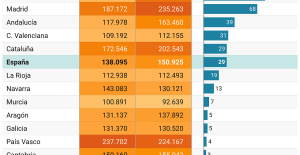 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade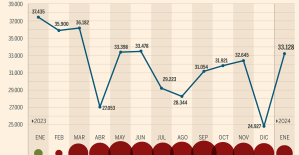 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella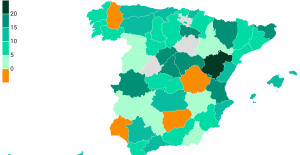 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Sale of Biogaran: The Republicans write to Emmanuel Macron
Sale of Biogaran: The Republicans write to Emmanuel Macron Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition
With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition Europeans: the schedule of debates to follow between now and June 9
Europeans: the schedule of debates to follow between now and June 9 These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Hand: Montpellier crushes Kiel and continues to dream of the Champions League
Hand: Montpellier crushes Kiel and continues to dream of the Champions League OM-Nice: a spectacular derby, Niçois timid despite their numerical superiority...The tops and the flops
OM-Nice: a spectacular derby, Niçois timid despite their numerical superiority...The tops and the flops Tennis: 1000 matches and 10 notable encounters by Richard Gasquet
Tennis: 1000 matches and 10 notable encounters by Richard Gasquet Tennis: first victory of the season on clay for Osaka in Madrid
Tennis: first victory of the season on clay for Osaka in Madrid


















