An american company has come to the last test of a possible coronavaccine.
Phase 3-the trial begins 27. July, and it is promising that the company Moderna is reached. The believe Jan Pravsgaard Christensen, who is a professor in the infektionsimmunologi of the University of Copenhagen.
- It means that the company has come through the first two nåleøjer, so the vaccine is safe, gives no side effects, and it can induce the immune response that should protect against the disease, he says.
A potential vaccine goes through three phases before eventually coming on the market. At the first stage, it is studied whether the product is dangerous for the people. It is not this, it goes on to the next phase. It makes the most.
In the second stage, it is tested whether the subjects develop either antibodies or so-called T-cells - both parts indicates that the product can give a positive effect. Here fail many products according to Jan Pravgaard Christensen.
- So, to go back to the desktop, and maybe start from scratch or at least modify its product significantly in order to get something out of it, he says.
A small group of products reaches to stage 3. The phase consists of the blindtest with two large groups of subjects. One group gets the product, while the other gets a placebo, in other words, a product without impact.
- In phase 3 it is studied whether the product actually has the effect you want. In other words, whether you can actually protect against being infected from the disease, or at least not get such a serious disease, as if one were not vaccinated, says Jan Pravsgaard Christensen.
When the groups have been respectively the product and the placebo, researchers examine whether those who have got the product, actually also is better protected against the virus, or at least get milder symptoms.
It is not a guarantee that the product ends up coming out on the market, although it has reached the final stage.
- There are many products where, in stage 3, discovers that it does not have the appropriate effect, " says the professor.
In some cases it turns out that only a small percentage of people that get the vaccine, are experiencing an effect. And so it will in most cases not make sense to go ahead with the product, because it becomes too difficult to sell.
The u.s. company Moderna stands to finish the experiment by 27. October 2022. It is expected, however, the results before then.
in Denmark, Too, are actors by developing possible coronavacciner. Both Statens Serum Institut and Copenhagen University in time, but it has not reached so far yet.

 Germany: the trial of an AfD leader, accused of chanting a Nazi slogan, resumes this Tuesday
Germany: the trial of an AfD leader, accused of chanting a Nazi slogan, resumes this Tuesday New York: at Columbia University, the anti-Semitic drift of pro-Palestinian demonstrations
New York: at Columbia University, the anti-Semitic drift of pro-Palestinian demonstrations What is Akila, the mission in which the Charles de Gaulle is participating under NATO command?
What is Akila, the mission in which the Charles de Gaulle is participating under NATO command? Lawyer, banker, teacher: who are the 12 members of the jury in Donald Trump's trial?
Lawyer, banker, teacher: who are the 12 members of the jury in Donald Trump's trial? What High Blood Pressure Does to Your Body (And Why It Should Be Treated)
What High Blood Pressure Does to Your Body (And Why It Should Be Treated) Vaccination in France has progressed in 2023, rejoices Public Health France
Vaccination in France has progressed in 2023, rejoices Public Health France Food additives suspected of promoting cardiovascular diseases
Food additives suspected of promoting cardiovascular diseases “Even morphine doesn’t work”: Léane, 17, victim of the adverse effects of an antibiotic
“Even morphine doesn’t work”: Léane, 17, victim of the adverse effects of an antibiotic Orthodox bishop stabbed in Sydney: Elon Musk opposes Australian injunction to remove videos on X
Orthodox bishop stabbed in Sydney: Elon Musk opposes Australian injunction to remove videos on X One in three facial sunscreens does not protect enough, warns L'Ufc-Que Choisir
One in three facial sunscreens does not protect enough, warns L'Ufc-Que Choisir What will become of the 81 employees of Systovi, a French manufacturer of solar panels victim of “Chinese dumping”?
What will become of the 81 employees of Systovi, a French manufacturer of solar panels victim of “Chinese dumping”? “I could lose up to 5,000 euros per month”: influencers are alarmed by a possible ban on TikTok in the United States
“I could lose up to 5,000 euros per month”: influencers are alarmed by a possible ban on TikTok in the United States Dance, Audrey Hepburn’s secret dream
Dance, Audrey Hepburn’s secret dream The series adaptation of One Hundred Years of Solitude promises to be faithful to the novel by Gabriel Garcia Marquez
The series adaptation of One Hundred Years of Solitude promises to be faithful to the novel by Gabriel Garcia Marquez Racism in France: comedian Ahmed Sylla apologizes for “having minimized this problem”
Racism in France: comedian Ahmed Sylla apologizes for “having minimized this problem” Mohammad Rasoulof and Michel Hazanavicius in competition at the Cannes Film Festival
Mohammad Rasoulof and Michel Hazanavicius in competition at the Cannes Film Festival Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars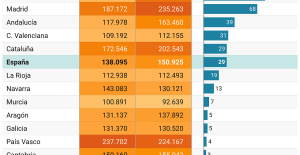 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade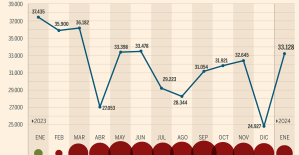 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella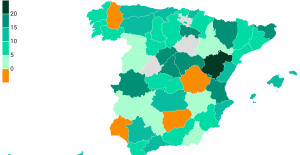 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition
With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition Europeans: the schedule of debates to follow between now and June 9
Europeans: the schedule of debates to follow between now and June 9 Europeans: “In France, there is a left and there is a right,” assures Bellamy
Europeans: “In France, there is a left and there is a right,” assures Bellamy These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Serie A: Bologna surprises AS Rome in the race for the C1
Serie A: Bologna surprises AS Rome in the race for the C1 Serie A: Marcus Thuram king of Italy, end of the debate for the position of number 9 with the Blues?
Serie A: Marcus Thuram king of Italy, end of the debate for the position of number 9 with the Blues? Milan AC-Inter Milan: Thuram and Pavard impeccable, Hernandez helpless… The tops and flops of the derby
Milan AC-Inter Milan: Thuram and Pavard impeccable, Hernandez helpless… The tops and flops of the derby Ligue 2: Auxerre leader, Bordeaux in crisis, play-offs... 5 questions about an exciting end of the season
Ligue 2: Auxerre leader, Bordeaux in crisis, play-offs... 5 questions about an exciting end of the season