It is one of the largest going to these fundraisers in Europe: the French Téléthon. Just in time for St. Nicholas day, the French TV broadcasts the 30-hour telethon to raise money for the development of new therapies for rare diseases. Also sick children to have their say. The impact: last year Alone, the Téléthon raised almost 70 million Euro.
The organization, AFM-Téléthon has become so large that it has its own research laboratory: Généthon. The approximately 200 employees focus mainly on basic research. And you made a breakthrough: they have developed a technique that used a Virus quasi as a Messenger, in the body of a sick people a defective Gene to be replaced.
With a Budget of 38 million euros, Généthon has the means to bring the invention to market. Therefore, the French completed in the year 2018 with the US Biotech company Avexis a license agreement for the use of the patents. Avexis to develop the means Zolgensma, the weakness against the most severe Form of genetically determined muscle, the spinal muscular atrophy (SMA), since this spring in the United States is approved.
Novartis is convinced of the prospects of the technology in such a way that the group took on Avexis in April for $ 8.7 billion. The money will be earned, therefore, Novartis set the price for the ground-breaking gene therapy Zolgensma with $ 2.1 million. This attracted international headlines, because this Product is the most expensive drug in the world.
Tricky price clause
However, the record high price could Slide. Because the license agreement between Avexis and Généthon, which is in the database of the U.S. securities and exchange Commission SEC available, contains an interesting clause. Under point 4.5 "French Patient Access" means: "The licensee (i.e., Avexis, d. ed.) will use reasonable efforts to provide to all licensed products for SMA in France, a price available to (...), which is not an obstacle for patient access."
thanks to this clause, the French have a lever to push the price for Zolgensma – at least in France. This could have consequences for the prices in countries such as Switzerland, where the authorities are laying down the reimbursement rates, among other things, the foreign prices of the draw.
The therapy with Zolgensma to about 2 million Swiss francs in costs. Photo: PD
the leaders of The Généthon answers, however, amazingly defensive on the question of whether you want to take advantage of the clause. "If the price for Zolgensma is too high or not, the need to find the French health authorities," says Généthon CEO Frédéric Revah. The clause was ultimately a kind of "potential opportunity for Appeal", should the authorities saw the price as too high.
In France, the Comité Economique des Produits de Santé (CEPS) specifies the medication price. Its Vice-Chairman Jean-Patrick Sales shall request only that Zolgensma is not yet approved in the EU. Therefore, "is Zolgensma not currently the subject of price negotiations in France". And Novartis, explains: "speculation about the possible pricing in individual countries, we do not comment on it."
alone of the French, has met with criticism
patient organisations, in the hope, however, to the clause in the contract: "I would hope that the French side uses the contract clause to achieve a sustainable and affordable price for Zolgensma, and that makes the result of the efforts publicly," says Nicole Gusset, President of SMA, Switzerland. "I'm afraid, however, that, as so often, only the list price in France is open to the public." The suspicion: France negotiated a hefty discount, it retains these, but for themselves.
The threat alone of the French gaining a foothold in France-criticism. "We are surprised that the leadership of AFM Téléthon, could consider to establish between the sick is a kind of discrimination, finally, the clause 4.5 relates to the contract, in French patients," criticized Luke Soubielle, President of the self-help organization Familles SMA France.
He suspects that the clause serve in the end only the image, in order to conceal that, thanks to the license agreement, Généthon forcefully Zolgensma is profiting. The contract provides that Généthon receives four million dollars at the time of contract signing. In addition, the laboratory receives payments from the Reach of certain milestones depend on the development, a total of 11 million dollars. For the third Généthon is involved with five percent of the sale proceeds.
practice test of the clause is from
Généthon-chief Revah defends himself: "My Problem is to find new resources." Therefore, the lab had to rely on such royalty income. In addition, the house could bring maturity without a Partner is not a drug to the market, it was too expensive. In most license agreements, Généthon would write such a clause into it, but so far, they had been never used.
Therefore, the Swiss refers to not-governmental organization of the Public Eye, the fact that the clause must pass the practical test. Nevertheless, the approach is worthy of imitation. "Many tax-funded inventions are sold without a guarantee that the resulting product is accessible or affordable," explains spokesperson Oliver Classen.
Only the fewest viewers of the French donation marathon, the Téléthon will probably have guessed that with your donations-developed innovations beibörsenkotierten at the end of big Pharma land.
Created: 26.11.2019, 19:37 PM

 Germany: the trial of an AfD leader, accused of chanting a Nazi slogan, resumes this Tuesday
Germany: the trial of an AfD leader, accused of chanting a Nazi slogan, resumes this Tuesday New York: at Columbia University, the anti-Semitic drift of pro-Palestinian demonstrations
New York: at Columbia University, the anti-Semitic drift of pro-Palestinian demonstrations What is Akila, the mission in which the Charles de Gaulle is participating under NATO command?
What is Akila, the mission in which the Charles de Gaulle is participating under NATO command? Lawyer, banker, teacher: who are the 12 members of the jury in Donald Trump's trial?
Lawyer, banker, teacher: who are the 12 members of the jury in Donald Trump's trial? What High Blood Pressure Does to Your Body (And Why It Should Be Treated)
What High Blood Pressure Does to Your Body (And Why It Should Be Treated) Vaccination in France has progressed in 2023, rejoices Public Health France
Vaccination in France has progressed in 2023, rejoices Public Health France Food additives suspected of promoting cardiovascular diseases
Food additives suspected of promoting cardiovascular diseases “Even morphine doesn’t work”: Léane, 17, victim of the adverse effects of an antibiotic
“Even morphine doesn’t work”: Léane, 17, victim of the adverse effects of an antibiotic Orthodox bishop stabbed in Sydney: Elon Musk opposes Australian injunction to remove videos on X
Orthodox bishop stabbed in Sydney: Elon Musk opposes Australian injunction to remove videos on X One in three facial sunscreens does not protect enough, warns L'Ufc-Que Choisir
One in three facial sunscreens does not protect enough, warns L'Ufc-Que Choisir What will become of the 81 employees of Systovi, a French manufacturer of solar panels victim of “Chinese dumping”?
What will become of the 81 employees of Systovi, a French manufacturer of solar panels victim of “Chinese dumping”? “I could lose up to 5,000 euros per month”: influencers are alarmed by a possible ban on TikTok in the United States
“I could lose up to 5,000 euros per month”: influencers are alarmed by a possible ban on TikTok in the United States Dance, Audrey Hepburn’s secret dream
Dance, Audrey Hepburn’s secret dream The series adaptation of One Hundred Years of Solitude promises to be faithful to the novel by Gabriel Garcia Marquez
The series adaptation of One Hundred Years of Solitude promises to be faithful to the novel by Gabriel Garcia Marquez Racism in France: comedian Ahmed Sylla apologizes for “having minimized this problem”
Racism in France: comedian Ahmed Sylla apologizes for “having minimized this problem” Mohammad Rasoulof and Michel Hazanavicius in competition at the Cannes Film Festival
Mohammad Rasoulof and Michel Hazanavicius in competition at the Cannes Film Festival Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars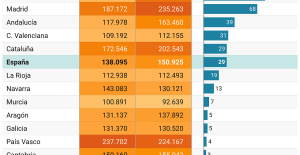 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade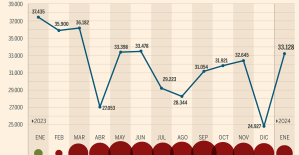 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella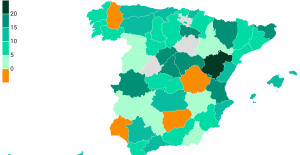 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition
With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition Europeans: the schedule of debates to follow between now and June 9
Europeans: the schedule of debates to follow between now and June 9 Europeans: “In France, there is a left and there is a right,” assures Bellamy
Europeans: “In France, there is a left and there is a right,” assures Bellamy These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Serie A: Bologna surprises AS Rome in the race for the C1
Serie A: Bologna surprises AS Rome in the race for the C1 Serie A: Marcus Thuram king of Italy, end of the debate for the position of number 9 with the Blues?
Serie A: Marcus Thuram king of Italy, end of the debate for the position of number 9 with the Blues? Milan AC-Inter Milan: Thuram and Pavard impeccable, Hernandez helpless… The tops and flops of the derby
Milan AC-Inter Milan: Thuram and Pavard impeccable, Hernandez helpless… The tops and flops of the derby Ligue 2: Auxerre leader, Bordeaux in crisis, play-offs... 5 questions about an exciting end of the season
Ligue 2: Auxerre leader, Bordeaux in crisis, play-offs... 5 questions about an exciting end of the season