Due to incorrect labelling of a still unknown number of packs of the anti-baby pill "Trigoa". Because some of them can be marked By blisters in the back the wrong way, threatened to unwanted pregnancies, told the Berlin state office for health and social Affairs (Lageso), as the competent Supervisory authority. According to the manufacturer, Pfizer, has a low number "of packages is concerned". In which of the länder, the affected batches were distributed to patients, is still unclear.
order taking
"Trigoa" according to Pfizer spokeswoman Susanne Straetmans, a three-phase preparation in which the pills are dosed differently to high and, accordingly, are different colored. Because of the dosage, the order of taking the tablets is not important.
callback period is extended
The Lageso, announced on late Friday afternoon, the women, the between 27. November and 3. December have received the contraceptive "Trigoa" from the affected batches X34106, X51153 and W98332, to give back to the drug through pharmacies.
On Monday, it was published on the Website of the Federal Institute for drugs and medical devices (BfArM) a note, which asks Doctors and pharmacists to warn women, "active", the between 27. November and 6. December could have redeemed a recipe for "Trigoa". The callback period is extended to three days.
The recall could also affect women, those on the pill prior to the 27. November has been prescribed, because prescriptions can be supplied for up to three months, it said in the from the BfArM published information.
Just a few Trigoa pills in circulation
How many packs which contain three by the recall affected batches, could not say a Pfizer spokeswoman Straetmans at the request of the daily mirror on Monday morning. Pfizer assume that "a small number of packs Germany was widely given to patients."
It was an older contraceptives with a very low market share, said Straetmans on Saturday. "Today, micro-pills are going through the same dose use." Since it was not on the receipt in the correct order.
More aboutdrug contamination recall of contaminated blood-pressure core prior to the completion of
Richard Friebeto reach The Lageso, was at the weekend for comment. (with dpa)

 Germany: Man armed with machete enters university library and threatens staff
Germany: Man armed with machete enters university library and threatens staff His body naturally produces alcohol, he is acquitted after a drunk driving conviction
His body naturally produces alcohol, he is acquitted after a drunk driving conviction Who is David Pecker, the first key witness in Donald Trump's trial?
Who is David Pecker, the first key witness in Donald Trump's trial? What does the law on the expulsion of migrants to Rwanda adopted by the British Parliament contain?
What does the law on the expulsion of migrants to Rwanda adopted by the British Parliament contain?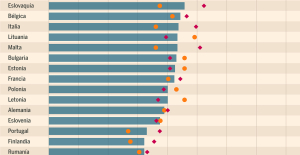 Spain is the country in the European Union with the most overqualified workers for their jobs
Spain is the country in the European Union with the most overqualified workers for their jobs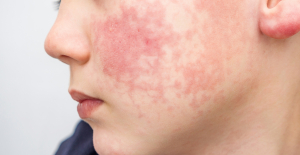 Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024
Parvovirus alert, the “fifth disease” of children which has already caused the death of five babies in 2024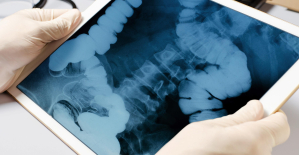 Colorectal cancer: what to watch out for in those under 50
Colorectal cancer: what to watch out for in those under 50 H5N1 virus: traces detected in pasteurized milk in the United States
H5N1 virus: traces detected in pasteurized milk in the United States Insurance: SFAM, subsidiary of Indexia, placed in compulsory liquidation
Insurance: SFAM, subsidiary of Indexia, placed in compulsory liquidation Under pressure from Brussels, TikTok deactivates the controversial mechanisms of its TikTok Lite application
Under pressure from Brussels, TikTok deactivates the controversial mechanisms of its TikTok Lite application “I can’t help but panic”: these passengers worried about incidents on Boeing
“I can’t help but panic”: these passengers worried about incidents on Boeing “I’m interested in knowing where the money that the State takes from me goes”: Bruno Le Maire’s strange pay slip sparks controversy
“I’m interested in knowing where the money that the State takes from me goes”: Bruno Le Maire’s strange pay slip sparks controversy 25 years later, the actors of Blair Witch Project are still demanding money to match the film's record profits
25 years later, the actors of Blair Witch Project are still demanding money to match the film's record profits At La Scala, Mathilde Charbonneaux is Madame M., Jacqueline Maillan
At La Scala, Mathilde Charbonneaux is Madame M., Jacqueline Maillan Deprived of Hollywood and Western music, Russia gives in to the charms of K-pop and manga
Deprived of Hollywood and Western music, Russia gives in to the charms of K-pop and manga Exhibition: Toni Grand, the incredible odyssey of a sculptural thinker
Exhibition: Toni Grand, the incredible odyssey of a sculptural thinker Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars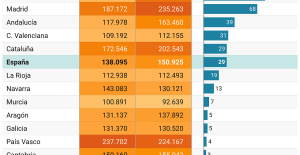 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade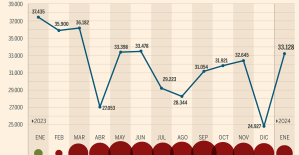 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella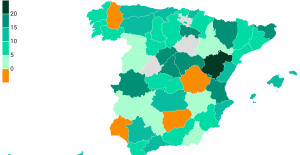 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Sale of Biogaran: The Republicans write to Emmanuel Macron
Sale of Biogaran: The Republicans write to Emmanuel Macron Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou
Europeans: “All those who claim that we don’t need Europe are liars”, criticizes Bayrou With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition
With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition Europeans: the schedule of debates to follow between now and June 9
Europeans: the schedule of debates to follow between now and June 9 These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Hand: Montpellier crushes Kiel and continues to dream of the Champions League
Hand: Montpellier crushes Kiel and continues to dream of the Champions League OM-Nice: a spectacular derby, Niçois timid despite their numerical superiority...The tops and the flops
OM-Nice: a spectacular derby, Niçois timid despite their numerical superiority...The tops and the flops Tennis: 1000 matches and 10 notable encounters by Richard Gasquet
Tennis: 1000 matches and 10 notable encounters by Richard Gasquet Tennis: first victory of the season on clay for Osaka in Madrid
Tennis: first victory of the season on clay for Osaka in Madrid


















