When Karl Lauterbach (SPD) announced the first delivery of Pfizer’s anti-corona drug Paxlovid at the end of 2021, the Federal Minister of Health sounded downright euphoric. The drug, which is intended to help prevent a severe course of the disease, is "extremely promising" and, in combination with the vaccines, could help make the Covid pandemic less frightening, Lauterbach praised the newly approved drug.
Similar to the Sars-CoV2 vaccines before, the federal government acted boldly and ordered one million packs of the virus inhibitor.
In the meantime, it has become apparent that this was obviously too bold: just over a year after the market launch in Germany, only 380,000 packs have been ordered, according to a request from WELT am SONNTAG to the Federal Ministry of Health.
The remaining 620,000 are still in stock at pharmaceutical wholesalers. It is not known how many of the delivered therapy units were actually prescribed to patients, because not only pharmacies, but also family doctor's surgeries, hospitals and care facilities can provide the drug in limited quantities. According to industry estimates, there are still around 120,000 packs of five daily doses in pharmacies, clinics and doctor's offices.
For the Federal Association of Pharmaceutical Wholesalers (Phagro), Paxlovid has long become a nuisance. "Due to the lack of medical prescriptions for Paxlovid, there is no need in the pharmacies, and the pharmaceutical wholesale trade is left with the Paxlovid procured by the federal government," it says there.
This initially also applies in the event that the drug reaches its expiry date. Because the federal government makes the decision about taking back or destroying the goods: "Pharmaceutical wholesalers are currently storing Paxlovid on a large scale free of charge and without remuneration for the federal government."
At least in view of the impending end of shelf life, relief is now looming. The Federal Ministry of Health announced that a “possible further extension to a total of 24 months” had been announced. The manufacturer Pfizer is informed that "since February 15, 2023, Paxlovid has a shelf life of 24 months".
The basis for this is the evaluation of further stability data, which is why the extension “also applies retrospectively to batches that have already been produced”. An official communication with a list of the corresponding batches and new expiry dates is currently being coordinated with the authorities and the Federal Ministry of Health.
Emanuel Wyler works at the Max Delbrück Center for Molecular Medicine in Berlin. The molecular biologist comments on a possible approval of Paxlovid in the WELT talk.
Source: WORLD / Nele Würzbach
In September 2022, the shelf life of Paxlovid in the EU was retrospectively extended from a year earlier to 18 months. Without another change, the first packs would have reached their expiry date at the end of May 2023.
It is not known how much the virus inhibitor has cost the federal government so far. The ministry announced that it had been agreed with the manufacturer not to disclose the purchase price.
In the USA, however, where the government ordered a total of 20 million packs in 2021, the price is known: It is around 530 dollars, the equivalent of 500 euros. However, Pfizer boss Albert Bourla had emphasized at the time that the USA would receive a special price because of the size of their order. For countries with smaller orders, the price is more in the order of around $700. If you apply these values, the federal government would have spent between 500 million and 660 million euros just for the purchase of Paxlovid.
The fact that the drug is only rarely used in Germany, despite sufficient availability, is primarily due to the reluctance of doctors to prescribe it. "Paxlovid is a highly effective drug for risk groups, but - just like any other preparation - should only be prescribed if there is a medical indication for it," says the German Association of General Practitioners. "For healthy, vaccinated people in their mid-40s, for example, a prescription is usually not necessary." Rather, as with any drug, the benefits and risks must be carefully weighed.
"Basically, over-therapy, which can potentially cause serious damage, should be avoided, as well as under-therapy, which could withhold possible help," confirms Martin Scherer, President of the German Society for General Medicine and Family Medicine (DEGAM). In addition, the list of possible interactions with other drugs is long, and clinical studies on this have so far been lacking.
In view of these restrictions, a significant increase in demand for Paxlovid is therefore unlikely, especially since the number of new infections has recently declined - even if the number of unreported cases has been high since the end of compulsory testing. "Basically, in the current situation, the higher the immunity in the population and the lower the number of infections in the risk groups, the less often Paxlovid has to be used," says the General Practitioners' Association. "Of course, how many units were ordered and whether they expire soon or not must not play a role in the prescription."
In retrospect, the neighboring country of Switzerland, with its approximately 8.7 million inhabitants, proceeded much more skilfully. Originally, 12,000 packs were ordered, of which around two-thirds have been delivered so far, according to the Federal Office of Public Health. And emphasizes, despite the small order quantity: "The supply of the Swiss population with Paxlovid was secured at all times."
"Everything on shares" is the daily stock exchange shot from the WELT business editorial team. Every morning from 7 a.m. with our financial journalists. For stock market experts and beginners. Subscribe to the podcast on Spotify, Apple Podcast, Amazon Music and Deezer. Or directly via RSS feed.

 Summoning the Iranian ambassador: how the dissemination of “fake news” forced Stéphane Séjourné to react
Summoning the Iranian ambassador: how the dissemination of “fake news” forced Stéphane Séjourné to react Germany: abortions should be authorized up to 12 weeks, concludes a commission launched by Olaf Scholz
Germany: abortions should be authorized up to 12 weeks, concludes a commission launched by Olaf Scholz Knife attack in Australia: who are the two French heroes congratulated by Macron?
Knife attack in Australia: who are the two French heroes congratulated by Macron? Faced with an anxious Chinese student, Olaf Scholz assures that not everyone smokes cannabis in Germany
Faced with an anxious Chinese student, Olaf Scholz assures that not everyone smokes cannabis in Germany Covid-19: everything you need to know about the new vaccination campaign which is starting
Covid-19: everything you need to know about the new vaccination campaign which is starting The best laptops of the moment boast artificial intelligence
The best laptops of the moment boast artificial intelligence Amazon invests 700 million in robotizing its warehouses in Europe
Amazon invests 700 million in robotizing its warehouses in Europe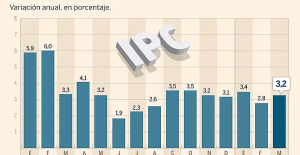 Inflation rises to 3.2% in March due to gasoline and electricity bills
Inflation rises to 3.2% in March due to gasoline and electricity bills Against drug trafficking, the mayor of Amsterdam advocates the regulation of cocaine
Against drug trafficking, the mayor of Amsterdam advocates the regulation of cocaine Hachette Livre removes Isabelle Saporta from management of Fayard
Hachette Livre removes Isabelle Saporta from management of Fayard Where is the MSC Aries, the ship boarded by Iran?
Where is the MSC Aries, the ship boarded by Iran? Denis Olivennes at Le Figaro: “CMI France discusses with Natacha Polony the future of Marianne”
Denis Olivennes at Le Figaro: “CMI France discusses with Natacha Polony the future of Marianne” Fernando Alonso signs the longest contract of his career for two fundamental reasons
Fernando Alonso signs the longest contract of his career for two fundamental reasons 2024 Candidates Chess Tournament: Relive the Crucial Nepo-Gukesh Game
2024 Candidates Chess Tournament: Relive the Crucial Nepo-Gukesh Game Borgo, by Stéphane Demoustier: locked outside
Borgo, by Stéphane Demoustier: locked outside Release of hostages and immediate ceasefire: at the Venice Biennale, the Israeli pavilion resonates with Gaza
Release of hostages and immediate ceasefire: at the Venice Biennale, the Israeli pavilion resonates with Gaza Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars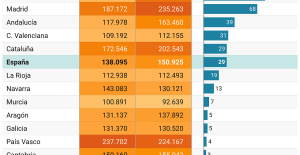 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade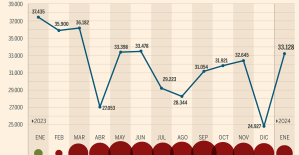 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Europeans: the schedule of debates to follow between now and June 9
Europeans: the schedule of debates to follow between now and June 9 Europeans: “In France, there is a left and there is a right,” assures Bellamy
Europeans: “In France, there is a left and there is a right,” assures Bellamy During the night of the economy, the right points out the budgetary flaws of the macronie
During the night of the economy, the right points out the budgetary flaws of the macronie Europeans: Glucksmann denounces “Emmanuel Macron’s failure” in the face of Bardella’s success
Europeans: Glucksmann denounces “Emmanuel Macron’s failure” in the face of Bardella’s success These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Champions League: in video, Barça's red card which leaves PSG alive
Champions League: in video, Barça's red card which leaves PSG alive Barça-PSG: in video, the goal of Ousmane Dembélé’s hope
Barça-PSG: in video, the goal of Ousmane Dembélé’s hope Surfing: eliminated from the pro tour, Kelly Slater close to saying goodbye
Surfing: eliminated from the pro tour, Kelly Slater close to saying goodbye Barcelona-PSG: in video, Vitinha’s goal which puts PSG in front
Barcelona-PSG: in video, Vitinha’s goal which puts PSG in front


















