For the new injections, recommended for people at risk of severe form, their entourage and caregivers, the HAS recommends, "preferably", "one of the three bivalent vaccines" - two developed by Pfizer / BioNTech, the third by Moderna - recently validated by the European Medicines Agency, according to a press release.
This recommendation applies regardless of the anti-Covid vaccine initially administered to the person.
It comes as Covid-19 contaminations have been increasing again in France for more than a week, suggesting a possible eighth wave of the epidemic at the end of the summer, the magnitude of which remains difficult to predict.
In France, as elsewhere in Europe, these bivalent vaccines are expected with the hope of having better shields against Sars-CoV2, before new dreaded waves this fall and winter.
These serums, which use messenger RNA technology like almost all the vaccines used so far, "are not new vaccines but vaccines adapted to circulating strains", underlined the HAS, drawing a parallel with the vaccines anti-flu.
These are the Moderna and Pfizer/BioNTech vaccines targeting the original strain of the virus and the Omicron BA.1 variant, and the Pfizer/BioNTech vaccine targeting the original strain and the BA subvariants .4 and BA.5 from Omicron.
Omicron and its sub-variants have been dominant throughout 2022, dethroning previous Alpha and Delta variants. The BA.4 and BA.5 subvariants are notably responsible for a new wave in Europe and the United States in recent months.
The opinion of the HAS, initially expected in early September, had been postponed to know all of the recommendations of the European regulator. It was taken "on the basis of the available data and in an epidemic context marked by the majority circulation of the BA.5 sub-variant", specified the HAS.
- Couple with the flu -
Compared to the original vaccines, "the expected clinical efficacy of these new bivalent vaccines is at least equivalent or even superior", and their tolerance is "identical", according to the HAS.
If it beats the vaccine booster again for the fall, the HAS insists on “the importance of protecting the populations most at risk”.
It recalls the two categories to be targeted for the new dose: the over 60s, and the under 60s at risk of a severe form of Covid-19 (people with comorbidities, pregnant women, immunocompromised people, children and adolescents with high risk), those around them ("cocooning strategy"), and professionals in the health and medico-social sector.
Anticipating the restart of the Covid-19 epidemic, with a new wave of uncertain "magnitude", the Minister of Health mentioned at the end of August "a vaccination campaign which will resume in the fall".
"The Ministry of Health and Prevention will take note of the opinion of the HAS, as since the start of the health crisis, and will decide on the vaccination strategy to be implemented on the basis of the recommendations", he said. -it was indicated Tuesday to the cabinet of François Braun.
For the new reminder campaign against Covid, the HAS maintains its recommendation to couple it with the annual flu vaccination, which will start on October 18. A concomitant injection or the same day of the two vaccines is possible, she recalls.
"In the immediate term and because the number of cases of infections has started to rise again for a few days", those most at risk are invited not to wait to receive their second reminder, if they have not yet received it. had.
Despite the recommendations, only about 30% of people over 60 received a second booster dose.
However, the original version of the vaccine (monovalent) is still effective in preventing serious forms, hospitalizations and deaths associated with the disease, hammer the health authorities. And it is the only possible for a first vaccination, notes the HAS.

 Rishi Sunak wants a tobacco-free UK
Rishi Sunak wants a tobacco-free UK In Africa, the number of millionaires will boom over the next ten years
In Africa, the number of millionaires will boom over the next ten years Iran's attack on Israel: these false, misleading images spreading on social networks
Iran's attack on Israel: these false, misleading images spreading on social networks Iran-Israel: David Cameron wants the G7 to impose “coordinated sanctions” on Iran
Iran-Israel: David Cameron wants the G7 to impose “coordinated sanctions” on Iran New generation mosquito nets prove much more effective against malaria
New generation mosquito nets prove much more effective against malaria Covid-19: everything you need to know about the new vaccination campaign which is starting
Covid-19: everything you need to know about the new vaccination campaign which is starting The best laptops of the moment boast artificial intelligence
The best laptops of the moment boast artificial intelligence Amazon invests 700 million in robotizing its warehouses in Europe
Amazon invests 700 million in robotizing its warehouses in Europe Solar panels: French manufacturer Systovi announces the cessation of its activities due to “Chinese dumping”
Solar panels: French manufacturer Systovi announces the cessation of its activities due to “Chinese dumping” Tesla: canceled in court, Musk's huge compensation plan will again be submitted to shareholders
Tesla: canceled in court, Musk's huge compensation plan will again be submitted to shareholders Two, three or a hundred euros: who are the most generous customers with tips?
Two, three or a hundred euros: who are the most generous customers with tips? Boeing safety examined in US Senate, after whistleblower's revelations
Boeing safety examined in US Senate, after whistleblower's revelations Immersion among the companions of the Liberation
Immersion among the companions of the Liberation Provence-Alpes-Côte d’Azur releases several hundred thousand euros for the promotion of the work of Marcel Pagnol
Provence-Alpes-Côte d’Azur releases several hundred thousand euros for the promotion of the work of Marcel Pagnol A palm of honor distinguishes Studios Ghibli for all of their work
A palm of honor distinguishes Studios Ghibli for all of their work Gaby, a new play by Pagnol adapted into a comic strip
Gaby, a new play by Pagnol adapted into a comic strip Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars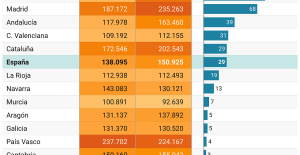 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade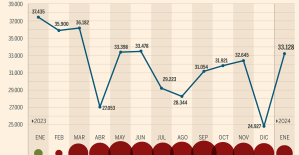 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella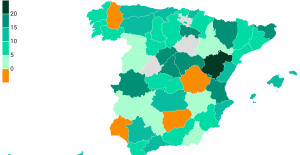 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? Europeans: the schedule of debates to follow between now and June 9
Europeans: the schedule of debates to follow between now and June 9 Europeans: “In France, there is a left and there is a right,” assures Bellamy
Europeans: “In France, there is a left and there is a right,” assures Bellamy During the night of the economy, the right points out the budgetary flaws of the macronie
During the night of the economy, the right points out the budgetary flaws of the macronie Europeans: Glucksmann denounces “Emmanuel Macron’s failure” in the face of Bardella’s success
Europeans: Glucksmann denounces “Emmanuel Macron’s failure” in the face of Bardella’s success These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Union Bordeaux Bègles-Clermont: at what time and on which channel to follow the Top 14 clash?
Union Bordeaux Bègles-Clermont: at what time and on which channel to follow the Top 14 clash? Football: Ada Hegerberg extends at OL until 2027
Football: Ada Hegerberg extends at OL until 2027 Basketball: suspended for life from NBA for fixing his match
Basketball: suspended for life from NBA for fixing his match Paris 2024 Olympic Games: boxer Estelle Mossely wants to parade on the Seine as a flag bearer
Paris 2024 Olympic Games: boxer Estelle Mossely wants to parade on the Seine as a flag bearer