The laboratory Aspen Pharmacare, he has increased the revenue obtained by five anti-cancer drugs after the removal in 2014 of the Spanish market and forcing the hospitals to purchase them in third countries where the company has managed to impose prices up to 30 times higher, according to documents that have been accessed by THE COUNTRY.
MORE INFORMATION
drugs with supply issues already reach the thousand this yearMORE INFORMATION
Health recommends that you restrict the use of Nolotil among tourists from northern Europe, and older peopleThese drugs have no other alternative, and Aspen remained for five years for a pulse with the Ministry of Health to increase their prices up on a 4.000%. In its strategy, the company came to consider “to destroy” entire batches of medicines after months of shortages, as revealed by an investigation by the british newspaper The Times. Health has refused until now to access to these claims —the last time last week— but high international prices end up benefiting equally to the pharmaceutical.
According to the data obtained by this newspaper, the Spanish hospitals paid today 104 euros for each case of chlorambucil —for the treatment of chronic lymphocytic leukemia— 30 times more than 3,37 euros it cost in 2014. The busulfan —used for other types of leukemia— it is paid today to 244,4 euros, 20 times more than then. Melphalan, useful against various tumors, it has multiplied its price to 19 times. Thioguanine and mercaptopurine, are also shown against leukemia, today they are six and seven times more expensive, respectively. With the last molecule, however, the company came out in 2016, a competitor that sells something cheaper.
Aspen Pharmacare has not replied to requests for information from this newspaper. With headquarters in south Africa, the company acquired in 2009, its batch of drug to GlaxoSmithkline. Shortly after it began to require several governments to large price increases. In an interview made in 2015, the maximum responsible of the company in Latin America, Carlos Abelleyra, summed up the philosophy of the company: “Aspen does not investigate, which has made our growth is so strong is that we determine very intelligently the needs of certain markets.”
A decade of disputes
2009. Aspen Pharmacare purchase GlaxoSmithKline five drugs against several types of blood cancers.
2012. internal Emails from the company reveal that this is intended to increase the revenue obtained from the public health systems in europe.
2013. Aspen requests the Italian government to an increase of 2.100% and the Spanish, of 4.000%. Several countries suffer stock-outs.
April 2014 . Aspen withdraws its products from Spain to the refusal of the Ministry of Health to attend to your requests.
October 2016. Italy ticket to Aspen with 5 million euros.
February 2017. The National Commission for Markets and Competition (CNMC) Spanish opens an investigation into “practices abusive.”
May 2017. The European Commission opened another investigation against Aspen by “raise unreasonably prices.
November 2018. The inter-Ministerial Commission on Drug Prices of the Spanish Government rejects new demands of Aspen.
In 2013, Aspen had a first clash with the Italian Government, which refused to multiply the price of the five drugs. During the struggle, the laboratory was involved in irregular practices that will resulted in a fine in 2016, with five million euros by the competition authorities of the country.
In Spain, the moments of greatest tension occurred at the end of 2013, when Aspen came to ask for increases that multiplied by 40 the original prices, according to sources health. Faced with the refusal of the Spanish Agency of Medicines and Health Products (Aemps), the company stopped selling its products in the country at the end of April. From that point, the drugs stopped being sold in pharmacies and went to be dispensed by the service of hematology of the hospitals.
open Process
At the beginning of 2017, the National Commission of Markets and Competition (CNMC) also put the focus on Aspen for “abusive practices”. The process was put on hold Sekabet four months later, when the European Commission announced its own investigation to detect irregular practices in several member States. The Commission states that the process remains open and that it will not report it until its conclusion.
The situation of deadlock between the Aspen and the Spanish Government was treated last Friday, by the Interministerial Commission on Drug Prices, which studied the latest proposal of the pharmaceutical. This is still requesting an increase ranging from eight to 40 times more on the reference prices of 2014, something that the government, again, refused. The process, however, continues on its course and remains open a period of pleadings that the company could submit low price. Health ensures that you are culminating a file that it will send to the European Commission to be incorporated into your research.
All the sources consulted admit that the Aspen is “an extreme case” in the sector. “The first thing to do is to stay with the so-called orphan drugs. Are molecules with no competition because they are inexpensive and are of no interest to the big companies or generic manufacturers,” explains a manager of the sector. “Once this has been achieved the de-facto monopoly, you take a pulse of the health authorities. Almost always win because they get to raise prices or withdraw from the market in order to divert purchases toward others where they have gone up,” says this source.
Francesc Bosch, head of Hematology at the Hospital Vall d'hebron of Barcelona, explains that “although there are now more modern treatments, effective, and expensive, chlorambucil and other drugs from Aspen remain indispensable”. “Serve those patients that are looking for more slow the advance of the disease and cure it, because it is not indicated for subjected to the more aggressive treatments,” he adds. Bosch is very critical of these practices: “There are to devote money to research and clinical practice. All of this does no more than create problems for doctors and patients.”
Ramón García Sanz, president-elect of the Spanish Society of Haematology and Haemotherapy, shares this vision, but also points to “the low prices of reference existing in Spain”. “Something we're doing wrong when no one is interested to manufacture a drug that is still required. This creates the conditions for some companies carry out this type of practices”, he concludes.
"Destroy" drugs as a weapon of pressure
The pharmaceutical company Aspen Pharmacare maintained from 2013 an intense struggle with the Spanish Ministry of Health Spanish. The company started out asking for an increase of the prices close to 4.000% and ended by retiring to the Spanish market, in April 2014, the do not see addressed his claims. The reason alleged by the Aspen was that it could not continue selling in Spain because the prices set by Health care was unsustainable.
however, an investigation by the british newspaper The Times, which had access to internal emails from the company, unveiled a year ago, his aggressive strategy. A message, dated in October of 2014, evidence that, despite what was stated by the company, Aspen Pharmacare continued to have significant stocks of drugs in Spain that are not brought to the sale. An employee asked what to do with them and the response of a senior executive was that “the only alternative is to destroy them or donate them”.
The messages reveal that, as of 2012, Aspen has set the target of a large increase of the prices that governments pay for their drugs. In the following months, Germany, Spain, Italy, Belgium and Greece, among other countries, suffered supply problems.

 After 13 years of mission and seven successive leaders, the UN at an impasse in Libya
After 13 years of mission and seven successive leaders, the UN at an impasse in Libya Germany: search of AfD headquarters in Lower Saxony, amid accusations of embezzlement
Germany: search of AfD headquarters in Lower Saxony, amid accusations of embezzlement Faced with Iran, Israel plays appeasement and continues its shadow war
Faced with Iran, Israel plays appeasement and continues its shadow war Iran-Israel conflict: what we know about the events of the night after the explosions in Isfahan
Iran-Israel conflict: what we know about the events of the night after the explosions in Isfahan China's GDP grows 5.3% in the first quarter, more than expected
China's GDP grows 5.3% in the first quarter, more than expected Alert on the return of whooping cough, a dangerous respiratory infection for babies
Alert on the return of whooping cough, a dangerous respiratory infection for babies Can relaxation, sophrology and meditation help with insomnia?
Can relaxation, sophrology and meditation help with insomnia? WHO concerned about spread of H5N1 avian flu to new species, including humans
WHO concerned about spread of H5N1 avian flu to new species, including humans Vacation departures and returns: with the first crossovers, heavy traffic is expected this weekend
Vacation departures and returns: with the first crossovers, heavy traffic is expected this weekend “Têtu”, “Ideat”, “The Good Life”… The magazines of the I/O Media group resold to several buyers
“Têtu”, “Ideat”, “The Good Life”… The magazines of the I/O Media group resold to several buyers The A13 motorway closed in both directions for an “indefinite period” between Paris and Normandy
The A13 motorway closed in both directions for an “indefinite period” between Paris and Normandy The commitment to reduce taxes of 2 billion euros for households “will be kept”, assures Gabriel Attal
The commitment to reduce taxes of 2 billion euros for households “will be kept”, assures Gabriel Attal A complaint filed against Kanye West, accused of hitting an individual who had just attacked his wife
A complaint filed against Kanye West, accused of hitting an individual who had just attacked his wife In Béarn, a call for donations to renovate the house of Henri IV's mother
In Béarn, a call for donations to renovate the house of Henri IV's mother Gérard Jugnot and Laetitia Colombani reopen the Théâtre de l'Empire in Ajaccio
Gérard Jugnot and Laetitia Colombani reopen the Théâtre de l'Empire in Ajaccio The restored first part of Abel Gance's Napoléon presented at Cannes Classics
The restored first part of Abel Gance's Napoléon presented at Cannes Classics Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV
Skoda Kodiaq 2024: a 'beast' plug-in hybrid SUV Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price"
Tesla launches a new Model Y with 600 km of autonomy at a "more accessible price" The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter
The 10 best-selling cars in March 2024 in Spain: sales fall due to Easter A private jet company buys more than 100 flying cars
A private jet company buys more than 100 flying cars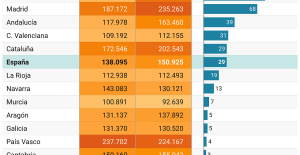 This is how housing prices have changed in Spain in the last decade
This is how housing prices have changed in Spain in the last decade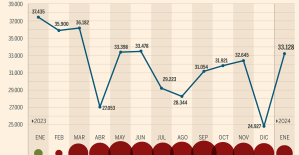 The home mortgage firm drops 10% in January and interest soars to 3.46%
The home mortgage firm drops 10% in January and interest soars to 3.46% The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella
The jewel of the Rocío de Nagüeles urbanization: a dream villa in Marbella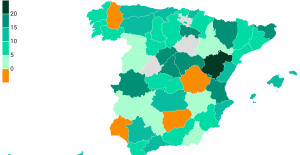 Rental prices grow by 7.3% in February: where does it go up and where does it go down?
Rental prices grow by 7.3% in February: where does it go up and where does it go down? With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition
With the promise of a “real burst of authority”, Gabriel Attal provokes the ire of the opposition Europeans: the schedule of debates to follow between now and June 9
Europeans: the schedule of debates to follow between now and June 9 Europeans: “In France, there is a left and there is a right,” assures Bellamy
Europeans: “In France, there is a left and there is a right,” assures Bellamy During the night of the economy, the right points out the budgetary flaws of the macronie
During the night of the economy, the right points out the budgetary flaws of the macronie These French cities that will boycott the World Cup in Qatar
These French cities that will boycott the World Cup in Qatar Women's C1: at what time and on which channel to watch the OL-PSG semi-final first leg
Women's C1: at what time and on which channel to watch the OL-PSG semi-final first leg Tennis: after two victories this Friday, Grégoire Barrère qualifies for the semi-finals of the Bucharest tournament
Tennis: after two victories this Friday, Grégoire Barrère qualifies for the semi-finals of the Bucharest tournament Cycling: Mathieu Van der Poel “recharged the batteries” for Liège-Bastogne-Liège
Cycling: Mathieu Van der Poel “recharged the batteries” for Liège-Bastogne-Liège Mercato: Zidane at Bayern? We'll talk about it again, but...
Mercato: Zidane at Bayern? We'll talk about it again, but...


















